首页 > 医疗资讯/ 正文
[摘要] 乳腺癌是女性发病率最高的恶性肿瘤,晚期乳腺癌患者的5年生存率不足20%。随着对乳腺癌发生、发展机制认识的深入,以及越来越多新药、新方案的问世,晚期乳腺癌患者的生存期不断延长。晚期乳腺癌的治疗进展推动了治疗共识的形成,但与此同时新的争议也在不断发生。本文基于2024年度关键临床研究证据,系统性梳理当前不同分子分型乳腺癌治疗的共识与争议,旨在为临床实践提供更全面的循证医学依据。针对人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)阳性晚期乳腺癌的一线治疗方案选择,尽管已经有CLEOPATRA研究结果使曲妥珠单抗和帕妥珠单抗联合紫杉类药物的治疗方案成为共识,但是PHILA研究结果提供了曲妥珠单抗和吡咯替尼联合紫杉类药物的新选择。两个研究亚组分析结果也为临床实践的差异化选择提供了参考。在晚期三阴性乳腺癌(triple-negative breast cancer,TNBC)的治疗中,对于程序性死亡蛋白配体-1(programmed death ligand-1,PD-L1)阳性患者,基于KEYNOTE-355、TORCHLIGHT研究,化疗联合程序性死亡蛋白-1 (programmed death-1,PD-1)抑制剂治疗已成为标准推荐。然而,免疫治疗的选择人群、PD-L1阳性标准及免疫治疗药物的对应选择仍存在争议。新的抗体药物偶联物(antibody-drug conjugate,ADC)联合PD-L1抑制剂治疗的早期研究显示,不依赖于PD-L1表达,在晚期TNBC患者一线治疗中获得迄今最长的超过1年的无进展生存期(progression-free survival,PFS),可能是未来一线方案的最佳选择。激素受体阳性、HER2阴性晚期乳腺癌患者,细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)4/6抑制剂联合内分泌治疗已成为指南推荐的标准一线治疗。但SONIA研究设计对指南推荐和有效药物先用的传统治疗理念提出了挑战,SONIA研究结果提示并非所有的激素受体阳性、HER2阴性晚期乳腺癌一线治疗方案都应该选择CDK4/6抑制剂,同时SONIA研究的局限性也猝然显现,即CDK4/6抑制剂治疗失败后的治疗选择目前还无标准推荐,但基于现有循证医学证据建议有靶点突变优选靶点药物治疗,若无靶点突变可选择ADC药物、内分泌治疗等。脑转移是临床治疗的难点,随着更多的药物包括靶向HER2的小分子酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)药物和大分子ADC药物被证实对HER2阳性脑转移治疗有效,如何将药物治疗与局部治疗进行有机地结合,是需要深入研究的焦点。ADC药物是目前研发的热点领域,随着ADC药物越来越多,如何选择是目前临床研究的重点。未来的研究需要关注创新药物研发、整合各种治疗手段,给予患者精准个体化治疗,延长患者的生存期。
[关键词] 晚期乳腺癌;共识;争议
[Abstract] Breast cancer remains the most prevalent malignancy in women, with a 5-year survival rate less than 20% in advanced stages. The deepening understanding of breast cancer pathogenesis and the emergence of novel therapeutic agents/regimens have progressively extended survival outcomes for advanced breast cancer patients. While therapeutic advancements have driven the formation of treatment consensus, new controversies continue to emerge. This article systematically reviews current consensus and controversies in managing different molecular subtypes of breast cancer based on pivotal 2024 clinical evidence, aiming to provide evidence-based guidance for clinical practice. For first-line treatment of human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer, the CLEOPATRA trial established the trastuzumab-pertuzumab-taxane regimen as standard care. However, the PHILA study proposed a new alternative combining trastuzumab, pyrotinib, and taxanes. Subgroup analyses from both trials provide valuable references for differentiated clinical decision-making. In triple-negative breast cancer (TNBC) management, KEYNOTE-355 and TORCHLIGHT trials established chemotherapy combined with programmed death-1 (PD-1) inhibitors as standard therapy for programmed death ligand-1 (PD-L1)-positive patients. Nevertheless, controversies persist regarding patient selection criteria, PD-L1 positivity thresholds, and optimal immunotherapy agents. Early-phase studies of antibody-drug conjugate (ADC)/PD-L1 inhibitor combinations demonstrated unprecedented progression-free survival (PFS) exceeding 12 months in the first-line TNBC treatment, independent of PD-L1 expression, potentially representing the future frontline regimen. For hormone receptor-positive/HER2-negative advanced breast cancer, cyclin-dependent kinase (CDK) 4/6 inhibitor-endocrine therapy combinations remain guideline-endorsed first-line treatment. The SONIA trial challenged conventional paradigms by demonstrating that not all patients required upfront CDK4/6 inhibitors. It also highlighted critical unresolved issues: no standard recommendations exist for post-CDK4/6 inhibitor therapy, though current evidence supports prioritizing targeted therapies for mutation-positive cases or ADC/endocrine therapies for mutation-negative scenarios. Brain metastasis management presents ongoing challenges. Emerging anti-HER2 agents, including tyrosine kinase inhibitor (TKI) and ADC with demonstrated intracranial efficacy, necessitate further investigation into optimal integration strategies with local therapies. ADC dominate therapeutic innovation, with current research prioritizing optimal sequencing strategies amidst expanding ADC options. Future directions should focus on novel drug development, multimodal treatment integration, and personalized precision therapies to prolong patient survival.
[Key words] Advanced breast cancer; Consensus; Controversies
晚期乳腺癌的治疗目的是在保证患者生活质量的前提下尽量延长生存期。随着对乳腺癌发生、发展机制认识的深入,以及越来越多新药、新方案研究结果的公布,晚期乳腺癌患者的生存期不断延长。总体而言,晚期乳腺癌的治疗进展推动了治疗共识的形成,但与此同时新的争议也在不断发生。本文对2024年度晚期乳腺癌治疗领域达成的主要共识与存在的争议进行梳理,旨在为乳腺癌的临床研究和疾病诊治提供参考。
1 HER2阳性晚期乳腺癌的治疗选择
1.1 治疗选择共识
靶向人表皮生长因子受体2( human epidermal growth factor receptor 2,HER2)药物的问世显著改善了HER2阳性晚期乳腺癌患者的预后。CLEOPATRA研究[1]比较了抗HER2抗体曲妥珠单抗+帕妥珠单抗与曲妥珠单抗单药分别联合紫杉类化疗药物的疗效,双抗治疗组显著延长了中位无进展生存期(median progression-free survival,mPFS)(18.5个月 vs 12.4个月)和中位总生存期(median overall survival,mOS)(57.1个月 vs 40.8个月)。PUFFIN研究[2-3]作为CLEOPATRA研究在中国的桥接试验,再次验证了曲妥珠单抗+帕妥珠单抗双靶组的显著生存优势。因此从2020年起,国内外指南一致推荐曲妥珠单抗+帕妥珠单抗双靶联合紫杉类药物作为HER2阳性晚期乳腺癌的标准一线治疗方案。
随着抗HER2药物的推陈出新,包括小分子酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)、抗体药物偶联物(antibody-drug conjugate,ADC)及新的一线治疗方案研究结果的公布,曲妥珠单抗+帕妥珠单抗双靶联合紫杉类药物方案已经不是唯一选择,如何选择一线治疗方案成为未来研究关注的焦点。
1.2 治疗选择争议
1.2.1 一线治疗方案靶向药物的选择策略
国内PHILA研究[4]针对HER2阳性晚期一线治疗患者,创新性选择大分子曲妥珠单抗联合小分子TKI再联合化疗与曲妥珠单抗联合化疗比较,对照组的选择与CLEOPATRA研究一致,结果显示,曲妥珠单抗联合吡咯替尼以及多西他赛相较于曲妥珠单抗联合多西他赛,显著改善了患者的PFS(24.3个月 vs 10.4个月),mPFS超过2年。PHILA研究结果提供了一线治疗方案的新选择。但是一线治疗方案中靶向药物选择大分子双抗还是大分子单抗与小分子TKI联合,目前尚无前瞻性随机对照研究比较其优劣。从研究亚组分析的结果可以初见端倪,为临床治疗选择提供帮助。CLEOPATRA研究[1]中有10%的患者既往曾用过曲妥珠单抗,探索性的亚组分析结果显示,经过曲妥珠单抗治疗的患者双靶治疗的PFS优于曲妥珠单抗单靶治疗(16.9个月 vs 10.4个月),但短于未经过曲妥珠单抗治疗的患者(21.6个月),OS相较于单靶组无明显延长(53.8个月 vs 46.6个月)。PHILA研究[4]纳入了15%既往经过曲妥珠单抗治疗的患者,亚组分析结果显示,曲妥珠单抗联合吡咯替尼的获益,经过曲妥珠单抗治疗组的风险比(hazard ratio,HR)明显低于未经过曲妥珠单抗治疗组(0.26 vs 0.46),提示对于既往经过曲妥珠单抗治疗的患者,曲妥珠单抗联合不同作用机制的小分子TKI药物吡咯替尼疗效可能更好。不同研究亚组分析结果为临床实践的差异化选择提供了参考。
此外,HER2阳性晚期乳腺癌患者最终约50%发生脑转移,脑转移患者的生存期明显缩短,是临床治疗的难点。现阶段一线治疗方案的选择也应将预防脑转移作为考量因素。CLEOPATRA研究[1]中,大分子抗体难以透过血脑屏障,两组的脑转移发生率相似(12.6% vs 13.7%)。TKI作为小分子药物,更容易透过血脑屏障,对脑转移治疗有效,PERMEATE研究[5]评估了吡咯替尼治疗HER2阳性脑转移的疗效,颅脑未接受过局部治疗的脑转移患者,颅内客观缓解率高达74.6%,mPFS为10.8个月,mOS达35.9个月,研究证实吡咯替尼治疗脑转移有效。因此PHILA研究[4]采用含吡咯替尼的方案,也许可以降低脑转移的发生率或推迟脑转移发生。
1.2.2 治疗选择的丰富
CLEOPATRA研究[1]和PHILA研究[4]聚焦于研究一线治疗方案中靶向药物的选择。JBCRG-M06/EMERALD研究[6]则聚焦于方案中化疗药物的选择,研究比较了HER2阳性晚期乳腺癌一线方案曲妥珠单抗+帕妥珠单抗双靶联合艾立布林与联合紫杉类药物,mPFS达到非劣效标准(14.0个月 vs 12.9个月),为一线方案提供了新的化疗药物选择。抗HER2药物除了大分子抗体、小分子TKI药物,目前新型ADC药物用于一线治疗的临床研究也在进行中,如德曲妥珠单抗(trastuzumab deruxtecan,T-DXd)和国产新型ADC药物SHR-A1811等都正在开展与现有标准治疗头对头比较的对照研究,ADC药物能否取代目前的标准治疗方案值得关注。
2 晚期三阴性乳腺癌(triple-negative breast cancer,TNBC)的治疗选择
2.1 治疗选择共识
既往晚期TNBC的一线标准治疗方案以化疗为主。CBCSG006研究[7]和GAP研究[8]证明了含铂类药物的方案(顺铂联合吉西他滨或白蛋白结合型紫杉醇)优于传统的紫杉类药物联合吉西他滨或卡培他滨,成为指南推荐的标准一线化疗方案。
基于TNBC具有肿瘤浸润淋巴细胞(tumor infiltrating lymphocyte,TIL)浸润水平高、程序性死亡蛋白配体-1(programmed death ligand-1,PD-L1)表达水平相对高、肿瘤突变负荷高等特点,预示其从免疫检查点抑制剂(immune checkpoint inhibitor,ICI)治疗中获益的可能性大[9]。Keynote-355研究[10]和TORCHLIGH研究[11]将晚期TNBC的治疗带入免疫治疗的时代。Keynote-355研究[10]结果显示,综合阳性评分(combined positive score,CPS)≥10的晚期TNBC,一线治疗采用帕博利珠单抗联合化疗较单纯化疗能显著改善mPFS(9.7个月 vs 5.6个月)及mOS(23.0个月 vs 16.1个月)。美国食品药品管理局(Food and Drug Administration,FDA)已批准帕博利珠单抗联合化疗作为CPS≥10的晚期TNBC的一线治疗方案,但该适应证在中国尚未获批。TORCHLIGHT研究[11]证实在PD-L1阳性(CPS≥1)的晚期TNBC的一线治疗中给予特瑞普利单抗联合白蛋白结合型紫杉醇较单药白蛋白结合型紫杉醇显著改善了mPFS(8.4个月 vs 5.6个月)和mOS(32.8个月 vs 19.5个月)。中国国家药品监督管理局(National Medical Products Administration,NMPA)已批准特瑞普利单抗联合白蛋白结合型紫杉醇作为CPS≥1的复发或转移性TNBC的一线治疗方案。
ICI包括程序性死亡蛋白-1(programmed death-1,PD-1)抑制剂和PD-L1抑制剂。PD-1抑制剂可阻断PD-1与PD-L1及PD-L2的结合,而PD-L1抑制剂主要阻断PD-L1与PD-1的结合,这种差异可能影响疗效和不良反应。Impassion 130研究[12-14]评估了PD-L1抑制剂阿替利珠单抗联合白蛋白结合型紫杉醇对比白蛋白结合型紫杉醇作为晚期TNBC的一线治疗方案的疗效,PD-L1阳性人群,阿替利珠单抗组有mPFS获益、OS延长,但该研究未达到设计的主要研究终点,即意向性治疗(intention-to-treat,ITT)人群和PD-L1阳性人群的PFS和OS双获益。Impassion 131研究[15]设计与Impassion 130类似,但所联合的化疗药物不是白蛋白结合型紫杉醇,而是紫杉醇,但阿替利珠单抗组也无PFS和OS获益。至此PD-L1抑制剂的两项研究均以失败告终。因此, PD-L1阳性的晚期TNBC患者,PD-1抑制剂联合化疗是推荐的一线治疗方案。
2.2 治疗选择争议
Keynote-355研究[10]和TORCHLIGH研究[11]奠定了PD-1抑制剂联合化疗为晚期TNBC的标准一线治疗方案的地位,但是这两项研究对于人群的选择并不一致。KEYNOTE-355研究[10]使用22C3抗体,定义PD-L1阳性为CPS≥10,研究入组患者的PD-L1阳性率为39%;TORCHLIGHT研究[11]使用JS311抗体,定义PD-L1阳性为CPS≥1,入组患者的PD-L1阳性率为56.4%。PD-L1检测用于筛选可能从免疫治疗中获益的患者。但不同研究对于PD-L1阳性的定义存在差异,这影响了临床试验结果的可比性和临床应用的标准化。临床实践中是否要根据特定抗体检测的PD-L1表达水平,选择相应的PD-1抑制剂给予患者治疗,仍有争议。
最新研究显示,ADC药物联合PD-L1抑制剂一线治疗晚期TNBC,疗效不受PD-L1表达水平影响。BEGONIA研究[16]中德达博妥单抗(datopotamab deruxtecan,Dato-DXd)联合度伐利尤单抗一线治疗晚期TNBC患者,mPFS达13.8个月,客观缓解率(objective response rate,ORR)高达79%。2024年欧洲肿瘤内科学会(European Society for Medical Oncology, ESMO)乳腺癌会议更新的MORPHEUS-pan BC研究[17]最新数据也显示,戈沙妥珠单抗(sacituzumab govitecan,SG)联合阿替利珠单抗作为一线方案治疗晚期TNBC,ORR为76.7%,mPFS为12.2个月,较单药SG组(5.9个月)延长1倍。两项ADC药物联合PD-L1抑制剂治疗方案,均不考虑PD-L1表达水平,取得了比Keynote-355和TORCHLIGH研究更好的mPFS。相应的Ⅲ期临床试验正在进行中,晚期TNBC一线治疗的未来可能是ADC药物联合免疫治疗,且无需考虑PD-L1表达水平。
3 激素受体阳性晚期乳腺癌的治疗选择
3.1 治疗选择共识
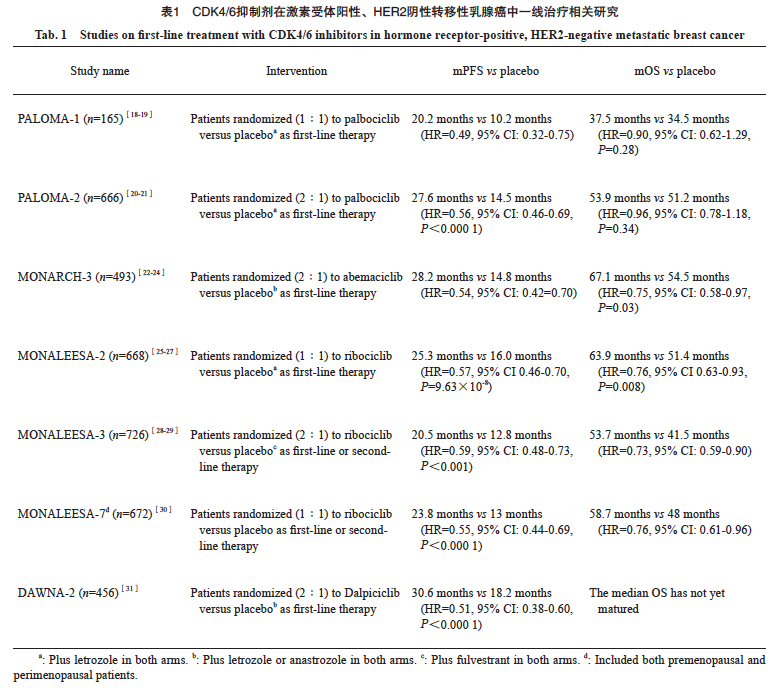
细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)4/6抑制剂联合内分泌治疗药物,已成为激素受体阳性晚期乳腺癌的一线治疗推荐。目前在国内上市的4种CDK4/6抑制剂分别开展了一线治疗的临床研究(表1),CDK4/6抑制剂联合内分泌治疗较单药治疗mPFS均有所获益。
3.2 治疗选择争议
3.2.1 是否所有患者均需一线选择CDK4/6 抑制剂
国内外指南中对于激素受体阳性/HER2阴性晚期乳腺癌,推荐的标准一线治疗方案均是CDK4/6抑制剂联合内分泌治疗,但是否所有患者都应如此选择值得商榷。SONIA研究[32]设计挑战了这一指南推荐,也挑战了有效药物先用的传统治疗理念,研究纳入了1 050例激素受体阳性/HER2阴性晚期乳腺癌患者,研究组一线用CDK4/6抑制剂联合非甾体芳香化酶抑制剂(nonsteroidal aromatase inhibitor,NSAI)治疗,进展后二线用氟维司群治疗(n=524),对照组一线用NSAI,进展后二线用CDK4/6抑制剂联合氟维司群,结果显示,两组的中位第二次无进展生存期(progression-free survival 2,PFS2)和OS均无显著差异,而一线使用CDK4/6抑制剂治疗组≥3级不良反应的发生例次明显高于对照组(2 782例次 vs 1 620例次)。另外两组患者的健康相关生活质量评分无显著差异,说明CDK4/6抑制剂的早期使用并未改善患者的生活质量。SONIA研究结果提示在临床实践中并非所有患者均需一线使用CDK4/6抑制剂,未来可以通过对生物标志物的深入研究精准选择患者。此外,研究中的研究组一线CDK4/6抑制剂治疗失败后,二线给予单药氟维司群治疗,这与指南共识推荐也不一致。目前对于CDK4/6抑制剂治疗失败后的选择,可以考虑靶向药物联合内分泌治疗、ADC药物等,但何为优选还存在争议。
3.2.2 CDK4/6抑制剂治疗失败后的选择
磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路(PAM通路)的过度激活是导致CDK4/6抑制剂耐药的主要机制之一。BYLieve研究[33]纳入PIK3CA突变、CDK4/6抑制剂治疗失败患者,PI3K抑制剂阿培利司联合氟维司群组的mPFS为7.3个月。CAPItello-291研究[34]入组708例激素受体阳性/HER2阴性晚期乳腺癌患者,其中496例患者既往接受过CDK4/6抑制剂治疗失败,在PI3K/AKT/PTEN基因改变的患者亚组中,AKT抑制剂卡匹色替(capivasertib)联合氟维司群组的mPFS为7.3个月,安慰剂联合氟维司群组为3.1个月,差异有统计学意义(HR=0.50,95% CI:0.38~0.65,P<0.001)。因此对于存在PAM通路改变的患者,CDK4/6抑制剂治疗失败后,PI3K抑制剂和AKT抑制剂应为优选方案。
对于CDK4/6抑制剂治疗失败后的患者,新型ADC药物也显示出良好疗效。DESTINY-Breast04研究[35]和DESTINY-Breast06研究[36]均入组HER2低表达人群,其中激素受体阳性患者中有70%~90%既往CDK4/6抑制剂治疗失败,T-DXd比单药化疗明显延长了PFS和OS。此外,靶向滋养层细胞表面抗原2(trophoblast cell-surface antigen 2,TROP-2)的ADC药物,SG和Dato-DXd在CDK4/6抑制剂治疗失败的激素受体阳性/HER2阴性晚期乳腺癌患者中也显示出显著疗效。TROPiCS-02研究[37]中,SG相较于化疗,PFS从4.0个月延长至5.5个月,OS从11.2个月延长至14.5个月。而在TROPION-Breast01研究[38]中,Dato-DXd治疗组的PFS为6.9个月,优于化疗组的4.9个月,疾病进展或死亡风险降低37%。
CDK4/6抑制剂治疗失败后的跨线治疗,也有不少研究进行了探索。最有代表性的是postMONARCH研究[39],这是一项前瞻性随机、双盲、安慰剂对照的Ⅲ期临床试验,入组患者分别有59%、33%和8%之前接受哌柏西利、瑞波西利和阿贝西利治疗失败,研究结果显示,阿贝西利联合氟维司群组的mPFS为6.0个月,而安慰剂组为5.3个月,尽管差异有统计学意义(P=0.02),但是CDK4/6抑制剂跨线使用较单药内分泌治疗仅延长了0.7个月。
尽管一些研究显示,PAM通路抑制剂、ADC药物、CDK4/6抑制剂再使用,对于CDK4/6抑制剂治疗失败后的患者均有疗效,但是如何进行个体化选择,还需要更深入的研究。
4 乳腺癌脑转移患者的治疗选择
4.1 治疗选择共识
2022年发表的中国临床肿瘤学会(Chinese Society of Clinical Oncology,CSCO)乳腺癌脑转移诊治专家共识[40]指出,乳腺癌患者确诊脑转移后,需要根据患者的一般情况、预期生存、颅外病灶的控制情况、脑转移灶的数目、部位、占位效应和手术风险等选择合理的局部治疗(放疗或手术)和支持治疗,并在此基础上,根据原发肿瘤的分子分型选择合适的全身抗肿瘤治疗。其中,外科手术能够切除脑转移灶、降低颅内压、缓解症状,对于占位明显的单发病灶效果显著。对于存在有限数目的多个病灶,但全身健康状况良好的患者,手术治疗也是优选方案。针对脑转移灶的放疗手段主要有全脑放疗(whole brain radiotherapy,WBRT)和立体定向放疗(stereotactic radiotherapy,SRS)。推荐对整体状态良好的无症状或症状轻微的HER2阳性乳腺癌脑转移患者可优先考虑系统性治疗,以延迟脑放疗的介入时机。一系列抗HER2的小分子TKI药物,如拉帕替尼、奈拉替尼、吡咯替尼和图卡替尼,均证实对HER2阳性乳腺癌脑转移患者有显著疗效(表2)。
除了小分子TKI药物治疗脑转移有显著疗效,研究显示,大分子新型ADC药物治疗脑转移也有良好疗效。DESTINY-Breast01、02、03研究[49]的探索性汇总分析表明,T-DXd在稳定性和活动性脑转移中均表现出较好的疗效,中枢神经系统(central nervous system,CNS)-PFS分别为12.3和18.5个月。DESTINY-Breast12研究[50]共招募了约500例HER2阳性转移性乳腺癌患者,其中脑转移队列是263例,mPFS达到17.3个月,12个月CNS-PFS率为58.9%。未经治疗的活动性脑转移亚组中,CNS-ORR可达80%以上。这些研究都验证了大分子ADC药物T-DXd也可作为HER2阳性乳腺癌脑转移患者的治疗选择。

4.2 治疗选择争议
随着更多的药物包括小分子TKI药物和大分子ADC药物被证实对脑转移治疗有效,如何将药物治疗与局部治疗进行联合是需要深入研究的焦点。BROPTIMA研究[51]是一项脑部放疗联合吡咯替尼及卡培他滨治疗HER2阳性乳腺癌脑转移的Ⅱ期临床试验,1年CNS-PFS率达到74.9%,中位CNS-PFS可达18个月,CNS-ORR为85%,放疗联合吡咯替尼和卡培他滨并未显著增加不良反应。该研究结果为乳腺癌脑转移特别是存在神经相关症状急需放疗的脑转移患者提供了新的治疗选择。目前也有小样本的回顾性研究分析了T-DXd联合脑放疗的疗效和安全性,总体安全可控,也为未来开展大样本量的研究提供了基础数据。这些研究聚焦于放疗和药物联合治疗,这种联合是同时还是序贯更好,尚未见相关研究报道,这些都是未来需要探索的方向。
药物治疗对于HER2阳性乳腺癌脑转移患者有效,但是HER2阴性乳腺癌脑转移患者还缺乏有效的治疗药物,治疗需求远未被满足。国内多中心开展的Ⅱ期临床试验[52]显示,优替德隆联合贝伐珠单抗治疗HER2阴性乳腺癌伴活动性脑转移患者,CNS-ORR为42.6%,mPFS为7.7个月,12个月OS率为74.4%。UTOBIA-BM研究[53]显示,优替德隆+依托泊苷+贝伐珠单抗的CNS-ORR为73%。基于上述研究数据美国FDA已授予优替德隆治疗乳腺癌脑转移孤儿药审评资格。这些研究为HER2阴性乳腺癌脑转移患者的治疗带来了希望。目前还有一系列临床试验聚焦于化疗联合抗血管生成药物、免疫治疗及ADC药物治疗HER2阴性乳腺癌脑转移,期待未来有更多的有效药物可供乳腺癌脑转移患者选择。
5 ADC时代的治疗选择共识与争议
近年来ADC药物成为研发热点。乳腺癌领域取得成功的ADC药物主要包括靶向HER2的恩美曲妥珠单抗(trastuzumab emtansine,T-DM1)和T-DXd,靶向TROP2的SG、芦康沙妥珠单抗和Dato-DXd。这些药物在晚期乳腺癌中均取得了明确疗效并获批适应证,目前在国内很多中心开展了联合免疫治疗、抗血管生成、内分泌治疗药物或化疗的临床研究,同时也在进行针对早期乳腺癌的临床研究。除目前研究较多的HER2和TROP-2靶点外,在乳腺癌领域还有很多其他靶点的ADC药物处于研发阶段,如锌转运蛋白LIV-1、脊髓灰质炎病毒受体相关蛋白4(Nectin cell adhesion molecule 4,Nectin-4)、HER3等,期待为ADC抗肿瘤领域增添新的选择。
随着ADC药物越来越多,如何优化选择是目前的研究热点。在法国开展的多中心回顾性研究[54]中,研究者评估了T-DXd和SG不同治疗顺序及序贯模式的疗效和安全性差异,研究共纳入179例接受SG序贯T-DXd或T-DXd序贯SG治疗的转移性乳腺癌患者,结果显示,无论序贯顺序如何,总人群的PFS2仅为2.7个月,表明使用相同载药类型(均为DNA拓扑异构酶Ⅰ抑制剂)的序贯治疗获益非常有限。即使在激素受体阴性人群中,先使用T-DXd后使用SG的治疗模式mPFS略好(mPFS为3.2个月),但整体获益仍不显著。此外,在后续ADC治疗中,即刻使用T-DXd或SG的患者的PFS2似乎比延迟使用者更长(3.1个月 vs 2.6个月),但差异较小。ADC药物的序贯使用顺序优化,也需要明确ADC药物耐药机制,才可能更精准地使用ADC药物。目前克服ADC耐药的手段主要有更换载药、研发双特异性抗体、联合其他治疗等。
总而言之,晚期乳腺癌是乳腺癌发展的特殊阶段,治疗过程复杂。治疗需要权衡多方面因素,包括患者的身体状况、病灶负荷、治疗手段、家庭经济状况等。一、二线治疗推荐的循证医学证据较充分,后线治疗暂时缺乏高水平证据。因此晚期乳腺癌的临床研究任重而道远。未来研究需要关注创新药物研发、整合各种治疗手段,给予患者个体化精准化治疗,延长患者的生存期。
第一作者:
王小波,博士,主治医师。
通信作者:
王涛,主任医师、教授。
作者贡献声明:
王小波:文章初稿撰写,文献检索;王涛:文章指导、修改及审核。
[参考文献]
[1] SWAIN S M, MILES D, KIM S B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a doubleblind, randomised, placebo-controlled, phase 3 study[J]. Lancet Oncol, 2020, 21(4): 519-530.
[2] XU B H, LI W, ZHANG Q Y, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): final analysis of a phase Ⅲ, randomized, doubleblind, placebo-controlled study[J]. Breast Cancer Res Treat, 2023, 197(3): 503-513.
[3] XU B H, LI W, ZHANG Q Y, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): a phase Ⅲ, randomized, double-blind, placebocontrolled study[J]. Breast Cancer Res Treat, 2020, 182(3): 689-697.
[4] MA F, YAN M, LI W, et al. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): randomised, double blind, multicentre, phase 3 trial[J]. BMJ, 2023, 383: e076065.
[5] YAN M, OUYANG Q C, SUN T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial[J]. Lancet Oncol, 2022, 23(3): 353-361.
[6] YAMASHITA T, SAJI S, TAKANO T, et al. Trastuzumab and pertuzumab in combination with eribulin mesylate or a taxane as first-line chemotherapeutic treatment for HER2-positive, locally advanced or metastatic breast cancer: results of a multicenter, randomized, non-inferiority phase 3 trial in Japan (JBCRG-M06/EMERALD)[J]. J Clin Oncol, 2024, 42(16_suppl): 1007.
[7] HU X C, ZHANG J, XU B H, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Oncol, 2015, 16(4): 436-446.
[8] WANG B Y, SUN T, ZHAO Y N, et al. A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer[J]. Nat Commun, 2022, 13(1): 4025.
[9] KEENAN T E, TOLANEY S M. Role of immunotherapy in triple-negative breast cancer[J]. J Natl Compr Canc Netw, 2020, 18(4): 479-489.
[10] CORTES J, RUGO H S, CESCON D W, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer[J]. N Engl J Med, 2022, 387(3): 217-226.
[11] JIANG Z F, OUYANG Q C, SUN T, et al. Toripalimab plus nab-paclitaxel in metastatic or recurrent triple-negative breast cancer: a randomized phase 3 trial[J]. Nat Med, 2024, 30(1): 249-256.
[12] EMENS L A, ADAMS S, BARRIOS C H, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis[J]. Ann Oncol, 2021, 32(8): 983-993.
[13] SCHMID P, RUGO H S, ADAMS S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2020, 21(1): 44-59.
[14] SCHMID P, ADAMS S, RUGO H S, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer[J]. N Engl J Med, 2018, 379(22): 2108-2121.
[15] MILES D, GLIGOROV J, ANDRÉ F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase Ⅲ trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer[J]. Ann Oncol, 2021, 32(8): 994-1004.
[16] WYSOCKI P J, MA C, PARK H, et al. Datopotamab deruxtecan (Dato-DXd)+durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase 1b/2 study[C]. Madrid: ESMO, 2023: 379MO.
[17] SCHMID P, LOI S, DE LA CRUZ-MERINO L, et al. Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: a phase Ⅰb/Ⅱ study of multiple treatment (tx) combinations in pts with locally advanced/metastatic BC (LA/mBC)[C]. Berlin: ESMO BC, 2024: abstract 181O.
[18] FINN R S, BOER K, BONDARENKO I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18)[J]. Breast Cancer Res Treat, 2020, 183(2): 419-428.
[19] WEDAM S, FASHOYIN-AJE L, BLOOMQUIST E, et al. FDA approval summary: palbociclib for male patients with metastatic breast cancer[J]. Clin Cancer Res, 2020, 26(6): 1208-1212.
[20] US Food And Drug Administration. Highlights of prescribing information: VERZENIO[R/OL]. (2017-09-28)[2025-01-08]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208716s000lbl.pdf.
[21] FINN R S, RUGO H S, DIERAS V C, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2- ABC): analyses from PALOMA-2[J]. J Clin Oncol, 2022, 40(17_suppl): LBA1003.
[22] CRISTOFANILLI M, TURNER N C, BONDARENKO I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, doubleblind, phase 3 randomised controlled trial[J]. Lancet Oncol, 2016, 17(4): 425-439.
[23] PATNAIK A, ROSEN L S, TOLANEY S M, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors[J]. Cancer Discov, 2016, 6(7): 740-753.
[24] GOETZ M, TOI M, HUOBER J, et al. Abstract GS01-12: MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer[J]. Cancer Res, 2024, 84(9_Supplement): GS01-12-GS01-12.
[25] INFANTE J R, CASSIER P A, GERECITANO J F, et al. A phase Ⅰ study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas[J]. Clin Cancer Res, 2016, 22(23): 5696-5705.
[26] SCHWARTZ G K, LORUSSO P M, DICKSON M A, et al. Phase Ⅰ study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1)[J]. Br J Cancer, 2011, 104(12): 1862-1868.
[27] HORTOBAGYI G N, STEMMER S M, BURRIS H A, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer[J]. N Engl J Med, 2022, 386(10): 942-950.
[28] SLAMON D J, NEVEN P, CHIA S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptorpositive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase Ⅲ randomized MONALEESA-3 trial: updated overall survival[J]. Ann Oncol, 2021, 32(8): 1015-1024.
[29] HORTOBAGYI G N, STEMMER S M, BURRIS H A, et al. Updated results from MONALEESA-2, a phase Ⅲ trial of firstline ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer[J]. Ann Oncol, 2019, 30(11): 1842.
[30] LU Y S, IM S A, COLLEONI M, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase Ⅲ randomized clinical trial[J]. Clin Cancer Res, 2022, 28(5): 851-859.
[31] ZHANG P, ZHANG Q Y, TONG Z S, et al. Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2023, 24(6): 646-657.
[32] SONKE G S, VAN OMMEN-NIJHOF A, WORTELBOER N, et al. Early versus deferred use of CDK4/6 inhibitors in advanced breast cancer[J]. Nature, 2024, 636(8042): 474-480.
[33] RUGO H S, LEREBOURS F, CIRUELOS E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study[J]. Lancet Oncol, 2021, 22(4): 489-498.
[34] OLIVEIRA M, RUGO H S, HOWELL S J, et al. Capivasertib and fulvestrant for patients with hormone receptor-positive, HER2-negative advanced breast cancer (CAPItello-291): patient-reported outcomes from a phase 3, randomised, doubleblind, placebo-controlled trial[J]. Lancet Oncol, 2024, 25(9): 1231-1244.
[35] YAMASHITA T, SOHN J H, TOKUNAGA E, et al. Trastuzumab deruxtecan versus treatment of physician’s choice in previously treated Asian patients with HER2-low unresectable/metastatic breast cancer: subgroup analysis of the DESTINY-Breast04 study[J]. Breast Cancer, 2024, 31(5): 858-868.
[36] BARDIA A, HU X C, DENT R, et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer[J]. N Engl J Med, 2024, 391(22): 2110-2122.
[37] XU B H, WANG S S, YAN M, et al. Sacituzumab govitecan in HR+HER2- metastatic breast cancer: the randomized phase 3 EVER-132-002 trial[J]. Nat Med, 2024, 30(12): 3709-3716.
[38] BARDIA A, JHAVERI K, KALINSKY K, et al. TROPIONBreast01: Datopotamab deruxtecan vs chemotherapy in pretreated inoperable or metastatic HR+/HER2- breast cancer[J]. Future Oncol, 2024, 20(8): 423-436.
[39] KALINSKY K, BIANCHINI G, HAMILTON E, et al. Abemaciclib plus fulvestrant in advanced breast cancer after progression on CDK4/6 inhibition: results from the phase Ⅲ postMONARCH trial[J]. J Clin Oncol, 2025, 43(9): 1101-1112.
[40] WANG T, CHEN J Y, YANG J, et al. CSCO expert consensus on the diagnosis and treatment of breast cancer brain metastasis[J]. Transl Breast Cancer Res, 2022, 3: 22.
[41] SALEEM A, SEARLE G E, KENNY L M, et al. Lapatinib access into normal brain and brain metastases in patients with HER-2 overexpressing breast cancer[J]. EJNMMI Res, 2015, 5: 30.
[42] LIN N U, DIÉRAS V, PAUL D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer[J]. Clin Cancer Res, 2009, 15(4): 1452-1459.
[43] BACHELOT T, ROMIEU G, CAMPONE M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study[J]. Lancet Oncol, 2013, 14(1): 64-71.
[44] PIVOT X, MANIKHAS A, ŻURAWSKI B, et al. CEREBEL (EGF111438): a phase Ⅲ, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer[J]. J Clin Oncol, 2015, 33(14): 1564-1573.
[45] AWADA A, COLOMER R, INOUE K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial[J]. JAMA Oncol, 2016, 2(12): 1557-1564.
[46] BORGES V F, FERRARIO C, AUCOIN N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial[J]. JAMA Oncol, 2018, 4(9): 1214-1220.
[47] MURTHY R, BORGES V F, CONLIN A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study[J]. Lancet Oncol, 2018, 19(7): 880-888.
[48] MURTHY R K, LOI S, OKINES A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer[J]. N Engl J Med, 2020, 382(7): 597-609.
[49] KOU L Q, CHEN X, XIE X L, et al. The efficacy and safety of trastuzumab deruxtecan (T-DXd) in HER2-expressing solid tumours: a single-arm meta-analysis[J]. Jpn J Clin Oncol, 2023, 53(8): 722-729.
[50] HARBECK N, CIRUELOS E, JERUSALEM G, et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial[J]. Nat Med, 2024, 30(12): 3717-3727.
[51] YANG Z Z, MENG J, MEI X, et al. Brain radiotherapy with pyrotinib and capecitabine in patients with ERBB2-positive advanced breast cancer and brain metastases: a nonrandomized phase 2 trial[J]. JAMA Oncol, 2024, 10(3): 335-341.
[52] YAN M, LV H M, LIU X L, et al. Utidelone plus bevacizumab for the treatment of HER2-negative breast cancer brain metastases (U-BOMB): a multicenter, single-arm phase Ⅱ study[J]. J Clin Oncol, 2024, 42(16_suppl): 1081.
[53] SHI Y, WANG P, ZHU Y, et al. Utidelone in combination with etoposide and bevacizumabin HER2-negative breast cancer with brain metastasis (UTOBIA-BM): a prospective, single-arm, phase Ⅱ trial[J]. Ann Oncol, 34(S2): S349-S350.
[54] POUMEAUD F, MORISSEAU M, CABEL L, et al. Efficacy of administration sequence: sacituzumab govitecan and trastuzumab deruxtecan in HER2-low metastatic breast cancer[J]. Br J Cancer, 2024, 131(4): 702-708.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)