首页 > 医疗资讯/ 正文
[摘要] 肿瘤转移是导致癌症患者高死亡率的主要原因。谷氨酰胺在肿瘤的恶性进展中发挥重要作用,近年来研究发现,谷氨酰胺与肿瘤转移密切相关,谷氨酰胺作为肿瘤细胞重要的碳源和氮源,不仅影响肿瘤细胞的增殖,还参与调控肿瘤细胞的迁移和侵袭。此外,谷氨酰胺代谢过程中的各种酶及转运体通过不同的信号转导通路参与肿瘤转移过程。本文综述近年来谷氨酰胺在肿瘤转移中作用的研究进展,并梳理有关治疗靶点,以期为临床治疗肿瘤转移提供新的策略。
[关键词] 谷氨酰胺代谢;肿瘤转移;上皮-间质转化;靶向治疗
[Abstract] Tumor metastasis is closely related to high mortality rate of cancer. It is well known that glutamine plays an important role in the malignant progression of cancer. Notably, as an important carbon and nitrogen donor, glutamine has been found to be closely related to tumor metastasis in recent years. Glutamine is not only involved in regulating the proliferation of tumor cells, but is also closely related to the migration and invasion of tumor cells. Furthermore, various enzymes along with transporters in the metabolism of glutamine are involved in the process of tumor metastasis through different signaling pathways. This review provided a summary of the role of glutamine in tumor metastasis in recent years and proposed therapeutic targets to provide new strategies for the clinical treatment of tumor metastases.
[Key words] Glutamine metabolism; Tumor metastasis; Epithelial-mesenchymal transition; Targeted therapy
肿瘤细胞的转移是由基因和蛋白表达或功能的改变引起的分子事件[1],包含一系列复杂的动态过程,高侵袭性的肿瘤具有浸润性,能够向周围间质扩散,经过血液循环寻找远处转移的部位,且在转移过程中还需适应营养供给和氧化应激的动态变化[2],之后在转移部位进一步地增殖。癌组织与癌旁组织相比,代谢发生了显著变化,且代谢变化成为驱动肿瘤转移的主要因素。谷氨酰胺是人体血清中含量最丰富的非必需氨基酸,并在肿瘤发生、发展过程中为癌细胞提供碳源和氮源,用于癌细胞的生物合成,为癌细胞提供能量,促进肿瘤的生长。有研究[3]表明,谷氨酰胺代谢在肿瘤细胞转移过程中发挥重要作用。本文综述近年来谷氨酰胺在肿瘤转移中作用及其机制的研究进展,并提出潜在的治疗靶点,以期为临床治疗肿瘤转移提供新的策略。
1 谷氨酰胺代谢概述
谷氨酰胺通过谷氨酰胺酶(glutaminase,GLS)生成谷氨酸和游离氨,接着谷氨酸经谷氨酸脱氢酶(glutamine dehydrogenase,GDH)催化生成α-酮戊二酸(α-ketoglutaric acid,α-KG),随后α-KG可进入三羧酸循环(tricarboxylic acid,TCA)参与糖代谢[4]。因此,谷氨酰胺可作为碳源补充TCA。此外,谷氨酰胺可经完全氧化生成腺苷三磷酸(adenosine triphosphate,ATP),作为氮源合成核苷酸和其他非必需氨基酸[5-6]。其代谢产物谷氨酸可作为合成谷胱甘肽的原料之一,与NADPH一起作为还原剂参与维持细胞内氧化还原稳态,利于细胞正常增殖(图1)。谷氨酰胺代谢重编程可作为肿瘤的代谢特征之一,在恶性增殖的肿瘤细胞中,谷氨酰胺代谢具有成瘾性,在没有外源性谷氨酰胺存在时无法存活[7]。谷氨酰胺代谢参与肿瘤细胞的生物前体合成,维持肿瘤细胞氧化还原稳态,并为肿瘤细胞提供能量,从而促进细胞增殖、迁移和侵袭。与谷氨酰胺代谢相关的酶,如GLS1/2[8]、谷氨酰胺合成酶(glutamine synthetase,GS)[9]、GDH[10]和谷氨酰胺转氨酶(glutamine transaminase,TG)[11],以及参与谷氨酰胺转运的蛋白质转运体,如SLC1A5[12]、SLC38A2[13]和SLC38A3[14]等,在肿瘤细胞内部或肿瘤微环境(tumor microenvironment,TME)中发挥调控肿瘤转移的作用。
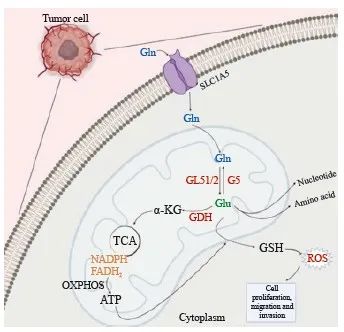
图1 谷氨酰胺代谢的主要途径
Fig. 1 The main pathway of glutamine metabolism
Glutamine enters the mitochondria of tumor cells via SL1A5 and is used to maintain the stability of intracellular redox levels through a series of reactions produce metabolites that affect the proliferation, invasion and migration of tumor cells. ROS: Reactive oxygen species.
2 谷氨酰胺代谢影响肿瘤转移的作用及机制
2.1 GLS与肿瘤转移
GLS是一种线粒体酶,分为GLS1和GLS2两种亚型,GLS1是一种线粒体基质蛋白,在转录阶段通过不同的剪切方式又可分为GAC(肝型)和KAG(肾型)两种亚型,GAC只存在于线粒体中,通过对肿瘤代谢物水平的调控参与肿瘤转移,而KAG主要在细胞质中发挥作用,KAG主要是通过介导蛋白质-蛋白质的相互作用,可能以非酶促的方式参与调控,因此GLS1在参与肿瘤细胞的代谢调控时,GAC亚型的表达抑制或促进在肿瘤代谢功能改变中占据主要地位。GLS1和GLS2虽然都能催化谷氨酰胺生成谷氨酸和游离的氨,但两者在肿瘤中的表达活性有明显差别。
GLS1在肿瘤中表现出高活性,该蛋白表达与肿瘤进展呈正相关[15]。GLS1在肝癌、胃癌、结直肠癌(colorectal cancer,CRC)、食管癌及肺癌等多种癌症中表达上调,促进肿瘤细胞增殖、侵袭和迁移,使用GLS1抑制剂BPTES、CB-893或经siRAN沉默形成siGLS1后,GLS1在肿瘤细胞中的表达活性减弱,可明显抑制肿瘤细胞迁移。最近研究[16-17]发现,在甲状腺乳头状癌中,GLS1高表达,雷帕霉素复合物1介导的信号通路被激活,ULK1被磷酸化失活,从而抑制细胞自噬,促进肿瘤细胞转移。多数情况下,细胞自噬水平增加不利于肿瘤发展,但是在肿瘤细胞后期,细胞自噬也可以通过清除破损细胞器,维持细胞内环境稳定来应对氧化应激,有利于肿瘤细胞生存,例如,在CRC中,GLS1高表达导致肿瘤细胞自噬水平增加,促进肿瘤进展,而GLS1缺失导致细胞自噬水平下降,与此同时细胞核中Nrf2蛋白表达降低,共同导致细胞内活性氧(reactive oxygen species,ROS)水平升高,从而抑制肿瘤转移,Nrf2蛋白作为一种转录因子通过调节下游的抗氧化基因来维持氧化还原平衡[18]。此外,在CRC中,GLS1可以作为缺氧诱导因子-1(hypoxia-inducible factor-1,HIF-1)的一个靶基因,HIF-1可与GLS1基因组中的一个位点结合,激活GLS1的表达来调节谷氨酰胺代谢,当GLS1缺乏可抑制缺氧诱导的肺定植和淋巴结转移。还有研究[19]显示,GLS1可与乳腺癌中的circHECTD1相互作用,促进肿瘤生长和转移。GLS1还可以通过增加ROS积累和抑制Wnt/β-catenin信号通路减弱干细胞特性,从而减少癌症干细胞(cancer stem cell,CSC)表型,CSC是一种能够自我更新并分化为其他不同类型的肿瘤细胞,具有干细胞的特性,致瘤潜能大[20]。因此癌症中存在CSC被认为是导致肿瘤复发、转移和获得化疗耐药性的重要原因[21],阻碍CSC表型形成能抑制肿瘤细胞增殖和迁移。
与GLS1不同的是,GLS2在多数情况下被认为是一种肿瘤抑制因子,且在肿瘤中的表达活性较低[22],其表达水平与肿瘤进展呈负相关。如在肝癌转移研究[23-24]中发现,GLS2的敲除导致肝癌细胞系发生肺转移的概率显著增加,GLS2主要是通过抑制小鸟苷三磷酸酶(guanosine triphosphatase,GTPase)Rac1的活性进而抑制肝癌细胞迁移和侵袭。同样有研究[25]报道,在肝癌中,GLS2表达通过稳定Dicer活性,诱导miR-34a的表达,接着降低snail的表达从而抑制上皮-间充质转化(epithelial-mesenchymal transition,EMT),进而抑制肿瘤转移。另外,在乳腺癌中,GLS2过表达可通过EGF-ERK-ZEB1-vimentin轴抑制EMT,从而抑制肿瘤进展[26]。
EMT与肿瘤的侵袭、转移及耐药密切相关,是肿瘤转移的起始过程也是关键步骤[27]。在此阶段,肿瘤上皮细胞丧失极性,成为间充质细胞,且更具有运动性,加速肿瘤细胞向远处转移,并使肿瘤获得CSC的特性[28]。EMT受到多种转录因子的调控,包括snail[29]、ZEB[30]、Slug[31]和TWIST[32]等。已有研究[33]证明,在胰腺癌细胞中,谷氨酰胺缺失引起的MEK/ERK信号通路和ATF4转录因子的激活能诱导Slug蛋白表达水平的增加,促进EMT,增强侵袭性,而抑制Slug蛋白表达,可以降低胰腺癌细胞侵袭和转移。
同样也有研究[34]证明,GLS2在乳腺癌、食管鳞状细胞癌(esophageal squamous cell carcinoma,ESCC)中作为致癌基因而存在,例如,甲基转移酶样因子3(methyltransferase like 3,METTL3)通过增强GLS2的表达促进ESCC的转移,下调GLS2可以减弱METTL3介导的肿瘤侵袭和迁移。与GLS1在多种癌症中的作用水平类似,GLS2的高水平表达与乳腺癌患者的不良预后相关。
2.2 GS与肿瘤转移
GS催化细胞内谷氨酸和游离氨生成谷氨酰胺,因此在氮代谢、氨解毒方面发挥作用,GS可催化谷氨酰胺分解代谢逆过程,作为唯一从头合成谷氨酰胺的酶,可以在细胞内通过合成谷氨酰胺来满足肿瘤细胞快速增殖的需求,因此在癌症转移中至关重要[35]。GS在胶质瘤[36]、卵巢癌[37]和乳腺癌[38]等癌症中表达上调,促进多种肿瘤的生长、增殖过 程。
GS在TME的调控中发挥重要作用,TME包括存在于肿瘤细胞外的细胞外基质、成纤维细胞、巨噬细胞、脂肪细胞及T淋巴细胞等,还包括血管的生成[39]。有研究[40]显示,肿瘤相关巨噬细胞的表型转化在多种类型肿瘤转移中发挥重要作用,并表现出EMT表型。肝癌细胞产生的抗炎因子促进巨噬细胞的M2型极化,促进肿瘤转移[41]。CRC细胞中,巨噬细胞同时存在M1和M2两种表型,其中M2表型发挥主要作用,导致CRC细胞侵袭、迁移和转移能力增强[42]。最近的研究[43]表明,GS活性下降能够介导肿瘤相关巨噬细胞表型转化,抑制肿瘤转移。
当巨噬细胞处于M0表型时,在炎症因子干扰素和其他激动剂的作用下转变为M1表型, M1表型表现出炎症反应,抑制血管生成,妨碍肿瘤转移过程,而GS的高表达促使M1表型巨噬细胞在白细胞介素(interleukin,IL)-4、IL-13和IL-10等作用下成为具有抗炎作用的M2表型[44]。GS增加细胞中琥珀酸水平,激活HIF-1使肿瘤细胞维持在M2表型,促进血管生成,增加T淋巴细胞的毒性作用,进而促进肿瘤转移(图2)。此外在肿瘤细胞中,癌基因c-myc被证明与谷氨酰胺成瘾有关,在口腔癌中,c-myc作为上游调节因子通过上调GLS和GS活性促进EMT,抑制c-myc的表达后明显减弱口腔癌细胞增殖和迁移能力[45]。

图2 GS在TME的巨噬细胞活化中的作用
Fig. 2 The role of GS in macrophage activation in TME
In TME, macrophages polarize from the M1 to M2 phenotype in response to GS, enhancing angiogenesis and T cell toxicity, which in turn promotes tumor metastasis. CAF: Cancer-associated fibroblast.
2.3 GDH与肿瘤转移
GDH用于催化谷氨酸生成α-KG,α-KG进入TCA参与能量供应和生物能合成。已有研究[46]表明,在肿瘤抑制因子LKB1缺失的肺癌细胞中,GDH1及下游产物α-KG通过CamKK2介导促进AMP活化蛋白激酶(AMP-activated protein kinase,AMPK)的磷酸化。AMPK被激活,触发能量代谢,抑制下游mTOR信号通路,从而增强凋亡抵抗,促进肺癌细胞转移[47]。GDH1通过酪氨酸135的磷酸化被表皮生长因子受体(epidermal growth factor receptor,EGFR)激活,通过CaMKIV信号转导与RSK2一起增强cAMP响应性元件结合蛋白活性,从而促进肺癌转移[48]。GDH还通过诱导STAT3介导的EMT来促进CRC细胞迁移。鉴于GDH的高表达与较差的临床结果有关,GDH可作为CRC转移的预后标志物之一[49]。有研究[50]显示,具有腺苷二磷酸(adenosine diphosphate,ADP)-核糖基化活性的线粒体Sirtuin 4通过促进GDH1的糖基化来抑制前列腺癌进展中的谷氨酰胺代谢途径。
2.4 TG与肿瘤转移
TG能催化蛋白质或多肽链内谷氨酰胺残基的水解和与其他氨基进行酰胺基的交换。TG蛋白家族包括TG1、TG2、TG3、TG4、TG5和TG6,其中TG2在参与并调控肿瘤转移中发挥关键作用。TG2在多种癌症中表达上调,如乳腺癌、CRC、肺癌、胰腺癌及卵巢癌等。
TG2同时具有酶促作用和非酶促作用,一方面TG2能催化Ca2+家族依赖蛋白的转酰胺化,发挥转氨酶的活性;另一方面,TG2具有GTPase、蛋白激酶和蛋白二硫化物异构酶活性[51],该活性在肿瘤细胞增殖、侵袭和转移的信号转导过程中发挥主要作用。此外,TG2在细胞外部和细胞内部都具有促进肿瘤转移的作用[52]。TG2与细胞膜表面的整联蛋白及细胞基质中的纤维连接蛋白相结合,诱导整联蛋白介导的细胞黏附的产生,从而促进肿瘤细胞转移,然而大部分TG2在细胞内部发挥作用,TG2可以与细胞内的HIF-1结合,进而刺激snail、Twist、ZEB等EMT调节因子的表达从而促进EMT,导致肿瘤细胞转移能力增强。然而这些作用都依赖于TG2的GTPase活性,很少需要转氨酶活性的参与。
在CRC中,TG直接调控EMT(snail和E-cadherin)和CSC(CD133和SOX2)相关蛋白的表达进而影响干细胞特性和肿瘤迁移及侵袭[53]。在肝癌中,癌症相关成纤维细胞(cancer-associated fibroblast,CAF)通过TG2诱导IL-6-IL6R-STAT3轴促进EMT,加速肝癌细胞侵袭和转移[54]。此外,也有研究[55]报道,真核延伸因子-2激酶通过上调TG2表达活性及整联蛋白介导的通路来增强胰腺癌细胞的EMT和迁移。在胃癌中,TG2高表达,通过介导ERK1/2活性参与胃癌细胞增殖和迁移[56]。TG2也可作为口腔鳞状细胞癌的生物标志物,在原发性肿瘤和转移性病灶中TG2的mRNA水平明显升高[57]。
2.5 谷氨酰胺转运蛋白与肿瘤转移
肿瘤细胞在转移过程中,需要消耗大量的氨基酸进入细胞,而其中包括许多外源性的非必需氨基酸,为满足外源性氨基酸进入肿瘤细胞的要求,氨基酸转运体发挥着关键作用。谷氨酰胺进入细胞需要谷氨酰胺转运蛋白的参与,如SLC1A5/ASCT2、SLC38A3等,其中SLC1A5是一种依赖Na+的跨膜转运蛋白,被认为在多种肿瘤细胞中担任主要的谷氨酰胺转运体角色,在胃癌、CRC、乳腺癌、非小细胞肺癌(non-small cell lung cancer,NSCLC)和肝癌等癌症中表达上调,能促进肿瘤细胞生长、增殖、迁移和EMT。在NSCLC中,SLC1A5可作为肿瘤淋巴结转移的不良预后标志,其表达水平与在原位瘤及转移性肿瘤中的表达呈正相关[58]。此外,在黏液表皮样癌所分离出来的一种具有高转移性的肿瘤组织MC3中,肿瘤抑制因子NDRG2通过抑制蛋白激酶B(protein kinase B,AKT)表达,减少对GSK3β的磷酸化,保持其活性,GSK3β通过诱导依赖Fbw7的c-myc的降解,导致c-myc活性降低,即从转录水平上抑制SCL1A5的表达,使肿瘤细胞依赖的谷氨酰胺分解减少,从而抑制EMT和肿瘤转移[59]。SLC38A3同样可通过激活PDK1/AKT信号通路减少EMT的上皮标志物E-cadherin的表达,增加间充质标志物N-cadherin的表达,从而促进EMT,导致NSCLC的细胞转移[60]。与原发性肿瘤相比,SLC38A2在转移性CRC组织中呈现高表达状态,SLC38A2的缺失有利于抑制肿瘤进展[61],并且与临床上三阴性乳腺癌患者的预后不良有关[62]。氨基酸逆向转运蛋白SLC7A5也对晚期CRC中肿瘤的转移和发展至关重要,在建立的转移性实验模型中, SLC7A5的缺失能显著延长模型小鼠生存期且减少肿瘤转移[63]。
3 靶向谷氨酰胺代谢干预肿瘤转移的治疗前景
目前处于临床前研究的靶向GLS抑制剂包括CB-839、6-重氮-5-氧-L-正亮氨酸、968和BPTES等,但是由于肿瘤细胞内代谢的复杂性,不只有谷氨酰胺代谢的存在,各种信号转导通路之间有重叠性,存在多个不同的靶点,因此谷氨酰胺代谢可以与其他临床药物联合使用,通过对多个代谢通路的抑制,增加抑瘤作用。已有研究[64]表明,其他靶向药物与GLS抑制剂联用具有良好的抗肿瘤效果。在CRC的治疗中,二甲双胍与利巴韦林、奥沙利铂和GLS抑制剂968联用具有较好的疗效[65]。968还可与自噬抑制剂氯喹联用,氯喹抑制968引起的细胞自噬,增强968的靶向抗肿瘤作用[66]。因此,实际应用中,可将谷氨酰胺代谢抑制剂与其他靶向抗肿瘤药物联合应用,增加治疗的可行性,为临床研究提供理论和实验基础,有希望在未来开发更多的抗肿瘤药物。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
刘雪柔:前期文献调研,文章初稿撰写;杨玉梅:文章框架整理;
赵倩:参考文献收集和插入;
荣翔宇:文章校样修改;
刘伟:文章返修内容核对,文献整理;
郑瑞洁:文章返修内容核对;
庞金龙:提供研究思路;
李娴:提供基金支持;
李姗姗:提供基金支持,文章审阅和修改。
[参考文献]
[1] REN L, RUIZ-RODADO V, DOWDY T, et al. Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma[J]. Cancer Metab, 2020, 8: 4.
[2] FARES J, FARES M Y, KHACHFE H H, et al. Molecular principles of metastasis: a hallmark of cancer revisited[J]. Signal Transduct Target Ther, 2020, 5(1): 28.
[3] BERGERS G, FENDT S M. The metabolism of cancer cells during metastasis[J]. Nat Rev Cancer, 2021, 21(3): 162-180.
[4] LEONE R D, ZHAO L, ENGLERT J M, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion[J]. Science, 2019, 366(6468): 1013-1021.
[5] WANG Y Y, BAI C S, RUAN Y X, et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia[J]. Nat Commun, 2019, 10(1): 201.
[6] KODAMA M, OSHIKAWA K, SHIMIZU H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer[J]. Nat Commun, 2020, 11(1): 1320.
[7] ADHIKARY G, SHRESTHA S, NASELSKY W, et al. Mesothelioma cancer cells are glutamine addicted and glutamine restriction reduces YAP1 signaling to attenuate tumor formation[J]. Mol Carcinog, 2023, 62(4): 438-449.
[8] JIANG B, ZHANG J, ZHAO G H, et al. Filamentous GLS1 promotes ROS-induced apoptosis upon glutamine deprivation via insufficient asparagine synthesis[J]. Mol Cell, 2022, 82(10): 1821-1835.e6.
[9] VILLAR V H, ALLEGA M F, DESHMUKH R, et al. Hepatic glutamine synthetase controls N5-methylglutamine in homeostasis and cancer[J]. Nat Chem Biol, 2023, 19(3): 292-300.
[10] SHANG M, CAPPELLESSO F, AMORIM R, et al. Macrophagederived glutamine boosts satellite cells and muscle regeneration[J]. Nature, 2020, 587(7835): 626-631.
[11] DORAI T, PINTO J T, DENTON T T, et al. The metabolic importance of the glutaminase Ⅱ pathway in normal and cancerous cells[J]. Anal Biochem, 2022, 644: 114083.
[12] YOO H C, PARK S J, NAM M, et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells[J]. Cell Metab, 2020, 31(2): 267-283.e12.
[13] GUO C S, YOU Z Y, SHI H, et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity[J]. Nature, 2023, 620(7972): 200-208.
[14] LIU R, HONG R X, WANG Y, et al. Defect of SLC38A3 promotes epithelial-mesenchymal transition and predicts poor prognosis in esophageal squamous cell carcinoma[J]. Chung Kuo Yen Cheng Yen Chiu, 2020, 32(5): 547-563.
[15] CHEN Y Y, TAN L, GAO J, et al. Targeting glutaminase 1 (GLS1) by small molecules for anticancer therapeutics[J]. Eur J Med Chem, 2023, 252: 115306.
[16] HAN T Y, WANG P C, WANG Y N, et al. FAIM regulates autophagy through glutaminolysis in lung adenocarcinoma[J]. Autophagy, 2022, 18(6): 1416-1432.
[17] YU Y, YU X H, FAN C L, et al. Targeting glutaminasemediated glutamine dependence in papillary thyroid cancer[J]. J Mol Med, 2018, 96(8): 777-790.
[18] LIU H Y, ZHANG H S, LIU M Y, et al. GLS1 depletion inhibited colorectal cancer proliferation and migration via redox/Nrf2/autophagy-dependent pathway[J]. Arch Biochem Biophys, 2021, 708: 108964.
[19] CAI J, CHEN Z Q, WANG J G, et al. circHECTD1 facilitates glutaminolysis to promote gastric cancer progression by targeting miR-1256 and activating β-catenin/c-Myc signaling[J]. Cell Death Dis, 2019, 10(8): 576.
[20] PASTUSHENKO I, BLANPAIN C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol, 2019, 29(3): 212-226.
[21] LI B H, CAO Y J, MENG G, et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/betacatenin pathway[J]. EBioMedicine, 2019, 39: 239-254.
[22] LUKEY M J, CLUNTUN A A, KATT W P, et al. Liver-type glutaminase GLS2 is a druggable metabolic node in luminalsubtype breast cancer[J]. Cell Rep, 2019, 29(1): 76-88.e7.
[23] SUZUKI S, VENKATESH D, KANDA H, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma[J]. Cancer Res, 2022, 82(18): 3209-3222.
[24] ZHANG C, LIU J, ZHAO Y H, et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis[J]. Elife, 2016, 5: e10727.
[25] KUO T C, CHEN C K, HUA K T, et al. Glutaminase 2 stabilizes dicer to repress snail and metastasis in hepatocellular carcinoma cells[J]. Cancer Lett, 2016, 383(2): 282-294.
[26] DIAS M M, ADAMOSKI D, DOS REIS L M, et al. GLS2 is protumorigenic in breast cancers[J]. Oncogene, 2020, 39(3): 690-702.
[27] BRABLETZ S, SCHUHWERK H, BRABLETZ T, et al. Dynamic EMT: a multi-tool for tumor progression[J]. EMBO J, 2021, 40(18): e108647.
[28] NALLASAMY P, NIMMAKAYALA R K, KARMAKAR S, et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis[J]. Gastroenterology, 2021, 161(6): 1998-2013.e7.
[29] XIE W, JIANG Q W, WU X J, et al. IKBKE phosphorylates and stabilizes Snail to promote breast cancer invasion and metastasis[J]. Cell Death Differ, 2022, 29(8): 1528-1540.
[30] LEE M Y, LIM S, KIM Y S, et al. DEP-induced ZEB2 promotes nasal polyp formation via epithelial-to-mesenchymal transition[J]. J Allergy Clin Immunol, 2022, 149(1): 340-357.
[31] LIANG Y P, CEN J J, HUANG Y, et al. CircNTNG1 inhibits renal cell carcinoma progression via HOXA5-mediated epigenetic silencing of Slug[J]. Mol Cancer, 2022, 21(1): 224.
[32] ANG H L, MOHAN C D, SHANMUGAM M K, et al. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds[J]. Med Res Rev, 2023, 43(4): 1141-1200.
[33] RECOUVREUX M V, MOLDENHAUER M R, GALENKAMP K M O, et al. Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer[J]. J Exp Med, 2020, 217(9): e20200388.
[34] CHEN X T, HUANG L L, YANG T T, et al. METTL3 promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression[J]. Front Oncol, 2021, 11: 667451.
[35] KIM G W, LEE D H, JEON Y H, et al. Glutamine synthetase as a therapeutic target for cancer treatment[J]. Int J Mol Sci, 2021, 22(4): 1701.
[36] ZHANG R, ZHU J C, HU H, et al. MicroRNA-140-5p suppresses invasion and proliferation of glioma cells by targeting glutamate-ammonia ligase (GLUL)[J]. Neoplasma, 2020, 67(2): 371-378.
[37] MENGA A, FAVIA M, SPERA I, et al. N-acetylaspartate release by glutaminolytic ovarian cancer cells sustains protumoral macrophages[J]. EMBO Rep, 2021, 22(9): e51981.
[38] XUAN D T M, WU C C, WANG W J, et al. Glutamine synthetase regulates the immune microenvironment and cancer development through the inflammatory pathway[J]. Int J Med Sci, 2023, 20(1): 35-49.
[39] QUAIL D F, JOYCE J A. Microenvironmental regulation of tumor progression and metastasis[J]. Nat Med, 2013, 19(11): 1423-1437.
[40] LIN Y X, XU J X, LAN H Y. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications[J]. J Hematol Oncol, 2019, 12(1): 76.
[41] WANG Y, GAO R F, LI J P, et al. Downregulation of hsa_ circ_0074854 suppresses the migration and invasion in hepatocellular carcinoma via interacting with HuR and via suppressing exosomes-mediated macrophage M2 polarization[J]. Int J Nanomedicine, 2021, 16: 2803-2818.
[42] WEI C, YANG C G, WANG S Y, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis[J]. Mol Cancer, 2019, 18(1): 64.
[43] PALMIERI E M, MENGA A, MARTÍN-PÉREZ R, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis[J]. Cell Rep, 2017, 20(7): 1654-1666.
[44] MENGA A, SERRA M, TODISCO S, et al. Glufosinate constrains synchronous and metachronous metastasis by promoting anti-tumor macrophages[J]. EMBO Mol Med, 2020, 12(10): e11210.
[45] WANG T, CAI B L, DING M C, et al. C-myc overexpression promotes oral cancer cell proliferation and migration by enhancing glutaminase and glutamine synthetase activity[J]. Am J Med Sci, 2019, 358(3): 235-242.
[46] BODINEAU C, TOMÉ M, MURDOCH P D S, et al. Glutamine, MTOR and autophagy: a multiconnection relationship[J]. Autophagy, 2022, 18(11): 2749-2750.
[47] JIN L T, CHUN J, PAN C Y, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer[J]. Mol Cell, 2018, 69(1): 87-99.e7.
[48] KANG J, CHUN J, HWANG J S, et al. EGFR-phosphorylated GDH1 harmonizes with RSK2 to drive CREB activation and tumor metastasis in EGFR-activated lung cancer[J]. Cell Rep, 2022, 41(11): 111827.
[49] LIU G J, ZHU J, YU M L, et al. Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients[J]. J Transl Med, 2015, 13: 144.
[50] MAO L, HONG X, XU L W, et al. Sirtuin 4 inhibits prostate cancer progression and metastasis by modulating p21 nuclear translocation and glutamate dehydrogenase 1 ADP-ribosylation[J]. J Oncol, 2022, 2022: 5498743.
[51] LEE H T, HUANG C H, CHEN W C, et al. Transglutaminase 2 promotes migration and invasion of lung cancer cells[J]. Oncol Res, 2018, 26(8): 1175-1182.
[52] ECKERT R L. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target[J]. Mol Carcinog, 2019, 58(6): 837-853.
[53] KANG S, OH S C, MIN B W, et al. Transglutaminase 2 regulates self-renewal and stem cell marker of human colorectal cancer stem cells[J]. Anticancer Res, 2018, 38(2): 787-794.
[54] JIA C C, WANG G Y, WANG T T, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma[J]. Int J Biol Sci, 2020, 16(14): 2542-2558.
[55] ASHOUR A A, GURBUZ N, ALPAY S N, et al. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion[J]. J Cell Mol Med, 2014, 18(11): 2235-2251.
[56] WANG X F, YU Z J, ZHOU Q, et al. Tissue transglutaminase-2 promotes gastric cancer progression via the ERK1/2 pathway[J]. Oncotarget, 2016, 7(6): 7066-7079.
[57] DING Y, LIU P F, ZHANG S S, et al. Screening pathogenic genes in oral squamous cell carcinoma based on the mRNA expression microarray data[J]. Int J Mol Med, 2018, 41(6): 3597-3603.
[58] CSANADI A, OSER A, AUMANN K, et al. Overexpression of SLC1a5 in lymph node metastases outperforms assessment in the primary as a negative prognosticator in non-small cell lung cancer[J]. Pathology, 2018, 50(3): 269-275.
[59] DING M C, BU X, LI Z H, et al. NDRG2 ablation reprograms metastatic cancer cells towards glutamine dependence via the induction of ASCT2[J]. Int J Biol Sci, 2020, 16(16): 3100-3115.
[60] WANG Y H, FU L, CUI M Q, et al. Amino acid transporter SLC38A3 promotes metastasis of non-small cell lung cancer cells by activating PDK1[J]. Cancer Lett, 2017, 393: 8-15.
[61] ZHANG D J, ZHAO L, SHEN Q, et al. Down-regulation of KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis in colorectal cancer[J]. Int J Cancer, 2017, 140(10): 2298-2309.
[62] MOROTTI M, ZOIS C E, EL-ANSARI R, et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer[J]. Br J Cancer, 2021, 124(2): 494-505.
[63] NAJUMUDEEN A K, CETECI F, FEY S K, et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer[J]. Nat Genet, 2021, 53(1): 16-26.
[64] INNAO V, RIZZO V, ALLEGRA A G, et al. Promising antimitochondrial agents for overcoming acquired drug resistance in multiple myeloma[J]. Cells, 2021, 10(2): 439.
[65] RICHARD S M, MARTINEZ MARIGNAC V L. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition[J]. J Cancer Res Ther, 2015, 11(2): 336-340.
[66] HALAMA A, KULINSKI M, DIB S S, et al. Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis[J]. Cancer Lett, 2018, 430: 133-147.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)