首页 > 医疗资讯/ 正文
[摘要] 背景与目的:肿瘤微血管生成是肿瘤生长、转移的重要基础,其特征包括血管新生、血管通透性增加及毛细血管结构异常等。微血管生成不仅影响肿瘤的供血和代谢,还与肿瘤的侵袭性、患者预后及治疗反应直接相关。动态对比增强磁共振成像(dynamic contrast-enhanced magnetic resonance imaging, DCE-MRI)是一种非侵入性的影像学技术,通过定量分析对比剂在肿瘤组织中的分布和动态变化,能够反映肿瘤的微血管密度(microvascular density,MVD)、通透性和血流灌注状态。本研究旨在通过深入分析直肠癌DCE-MRI定量分析参数与微血管生成的关系,进一步明确其在直肠癌诊断和治疗中的应用价值,并推动该技术在临床实践中的普及和优化。方法:选取2021年1月—2024年6月新乡市中心医院收治的348例拟行手术治疗的直肠癌患者,并收集直肠癌组织标本和癌旁组织(距离肿瘤边缘>5 cm)。本研究经新乡市中心医院医学伦理委员会审批通过(批号:2021-144-01K)。比较癌组织、癌旁组织中的DCE-MRI定量分析参数[速率常数(rate constant,Kep)、容积转运常数(volume transfer constant,Ktrans)、细胞外间隙对比剂容积分数(extravascular extracellular volume fraction,Ve)]和MVD。对比不同分化程度、临床分期直肠癌患者的DCE-MRI定量分析参数和MVD。采用Spearman相关系数分析直肠癌患者的DCE-MRI参数与分化程度、临床分期及MVD的相关性。结果:直肠癌组织的Kep值、Ktrans值、Ve值及MVD均高于癌旁组织(P<0.05)。低分化和中分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于高分化直肠癌患者(P<0.05),低分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于中分化直肠癌患者(P <0.05)。Ⅱ期、Ⅲ期和Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅰ期直肠癌患者(P<0.05),Ⅲ期和Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅱ期直肠癌患者(P<0.05),Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅲ期直肠癌患者(P<0.05)。直肠癌患者的Kep值、Ktrans值及Ve值与分化程度均呈负相关(r=-0.683、-0.743、-0.721,P<0.05),直肠癌患者的Kep值、Ktrans值及Ve值与临床分期均呈正相关(r=0.764、0.703、0.814,P<0.05),直肠癌患者的Kep值、Ktrans值及Ve值与MVD均呈正相关(r=0.812、0.754、0.835,P<0.05)。结论:DCE-MRI参数与直肠癌分化程度、临床分期及微血管生成均相关。
[关键词] 直肠癌;动态对比增强磁共振成像;定量参数;分化程度;临床分期;微血管生成
[Abstract] Background and purpose: Tumor microangiogenesis is an important basis for tumor growth and metastasis, and its characteristics include angiogenesis, increased vascular permeability and abnormal capillary structure. Microangiogenesis not only affects the blood supply and metabolism of tumor, but also is directly related to the invasion, prognosis and treatment response of tumor. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a non-invasive imaging technique. By quantitatively analyzing the distribution and dynamic changes of contrast agents in tumor tissues, it can reflect the microvascular density (MVD), permeability and blood perfusion of tumors. The purpose of this study was to further clarify the application value of DCE-MRI in the diagnosis and treatment of rectal cancer by in-depth analysis of the relationship between quantitative analysis parameters of rectal cancer and microangiogenesis, and to promote the popularization and optimization of this technology in clinical practice. Methods: A total of 348 patients with rectal cancer who were scheduled for surgical treatment in Xinxiang Central Hospital from January 2021 to June 2024 were selected, and rectal cancer tissue specimens and adjacent tissues (> 5 cm away from tumor margin) were collected. This study was approved by the medical ethics committee of Xinxiang Central Hospital (approval number: 2021-144-01K). The quantitative analysis parameters of DCE-MRI [Rate constant (Kep), volume transport constant (Ktrans), volume fraction of contrast agent in extracellular space (VE)] and MVD in cancer tissues and adjacent tissues were compared. The quantitative analysis parameters and MVD of DCE-MRI in rectal cancer patients with different differentiation degrees and clinical stages were compared. Spearman correlation was used to analyze the correlation between DCE-MRI parameters and differentiation degree, clinical stage and MVD in patients with rectal cancer. Results: The values of Kep value, Ktrans value, Ve value and MVD were higher in rectal cancer tissues than in adjacent tissues (P<0.05). The Kep value, Ktrans value, Ve value and MVD of patients with low differentiated and middle differentiated rectal cancer were higher than those of patients with high differentiated rectal cancer (P<0.05). The values of Kep value, Ktrans value, Ve value and MVD of patients with low differentiated rectal cancer were higher than those of patients with middle differentiated rectal cancer (P<0.05). The Kep value, Ktrans value, Ve value and MVD of patients with stage Ⅱ, Ⅲ and Ⅳ rectal cancer were higher than those of patients with stage Ⅰ rectal cancer (P<0.05). The Kep value, Ktrans value, Ve value and MVD of patients with stage Ⅲ and Ⅳ rectal cancer were higher than those of patients with stage Ⅱ rectal cancer (P<0.05). The Kep value, Ktrans value, Ve value and MVD of patients with stage Ⅳ rectal cancer were higher than those of patients with stage Ⅲ rectal cancer (P<0.05). The Kep value, Ktrans value and Ve value of rectal cancer patients were negatively correlated with the differentiation degree (r=-0.683, -0.743, -0.721, P<0.05). The Kep value, Ktrans value and Ve value of rectal cancer patients were positively correlated with clinical stage (r=0.764, 0.703, 0.814, P<0.05). The Kep value, Ktrans value and Ve value of rectal cancer patients were positively correlated with MVD (r=0.812, 0.754, 0.835, P<0.05). Conclusion: DCE-MRI parameters are related to the differentiation degree, clinical stage and microangiogenesis of rectal cancer.
[Key words] Rectal cancer; Dynamic contrast-enhanced magnetic resonance imaging; Quantitative parameters; Degree of differentiation; Clinical staging; Microangiogenesis
直肠癌为临床常见的消化道恶性肿瘤,具有发病率高、死亡率高的特点[1]。近年来,直肠癌的发病率呈逐年上升趋势,且趋于年轻化[2]。新生血管可给肿瘤组织输送营养物质和氧气,释放一些可促进肿瘤细胞增殖的生长因子促进其生长,并通过癌组织中大量的分支血管促进肿瘤转移的发生[3]。因此对微血管生成进行准确的评估,对临床治疗方案的选择及患者预后改善具有重大意义。微血管密度(microvascular density,MVD)是目前反映肿瘤血管生成的“金标准”,但MVD测量方法创伤大,且无法长期动态、重复观察[4],在临床应用中具有一定的局限性。因此,急需寻找一种无创、快捷的检查方法对肿瘤血管生成进行准确的评估。动态对比增强磁共振成像(dynamic contrast-enhanced magnetic resonance imaging,DCE-MRI)定量分析通过动态监测低分子量对比剂在组织中的吸收、代谢等药代动力学过程,可在细胞分子功能水平上反映组织血流灌注、血管分布等信息[5];目前DCE-MRI已用于乳腺癌、脑胶质瘤等多种肿瘤的研究中[6-7],但DCE-MRI定量分析参数与直肠癌患者微血管生成的关系尚不清楚。因此,本研究旨在通过深入分析直肠癌DCE-MRI定量分析参数与微血管生成的关系,进一步明确其在直肠癌诊断和治疗中的应用价值,并推动该技术在临床实践中的普及和优化。
1 资料和方法
1.1 临床资料
选取2021年 1月—2024年 6月新乡市中心医院收治的348例直肠癌患者作为研究对象,其中男性186例,女性162例,年龄24~73岁,平均(53.61±11.42)岁;体质量指数为19.36~27.51 kg/m2,平均(22.18±4.36)kg/m2。本研究经新乡市中心医院医学伦理委员会审批通过(批号:2021-144-01K)。
入组标准:① 符合直肠癌诊断标准[8],并经病理学检查确诊;② 年龄>18岁;③ DCE-MRI检查前未接受放疗、化疗等治疗;④ 均接受手术治疗;⑤ 患者对本研究知情同意并签署知情同意书。排除标准:① 重要脏器功能不全者;② 合并其他部位恶性肿瘤者;③ 合并血液系统或免疫系统疾病者;④ 对比剂过敏者;⑤ 无法配合或耐受DCE-MRI检查者。
1.2 DCE-MRI检查及图像处理
检查前6~8 h禁食;取仰卧位,用核磁共振扫描仪(型号:Discovery MR750W HD 3.0 T,美国GE公司)进行扫描,扫描范围包括整个盆腔;扫描序列包括常规MRI扫描(轴位T1WI,轴位、冠状位、矢状位T2WI)、弥散加权成像(diffusion weighted imaging,DWI)及DCE-MRI横断面扫描,共采集50个时相,于第3个时相扫描开始时注入对比剂钆喷酸葡胺,扫描总时长约540 s;扫描时先用大扫描视野(field of view,FOV)对盆腔的总体情况进行评估;随后使用小FOV、薄层厚的高分辨率MRI对病变区进行重点扫描;将采集的DCE-MRI数据传输至工作站,进行定量参数分级,产生伪彩图,将其与常规MRI结合确定感兴趣区(region of interest,ROI),肿瘤及瘤旁ROI的选取均用ImageJ软件基于影像学特征进行自动勾画;由2名影像科医师进行双盲阅片测定,软件生成ROI内定量参数,包括速率常数(rate constant,Kep)、容积转运常数(volume transfer constant,Ktrans)和细胞外间隙对比剂容积分数(extravascular extracellular volume fraction,Ve)。
1.3 分化程度
直肠癌组织的分化程度依据病理学检查[9]结果分为高分化、中分化和低分化。
1.4 临床分期
依据美国癌症学会分期标准[10]将直肠癌患者分为Ⅰ期(肿瘤侵犯黏膜下层)、Ⅱ期(肿瘤侵犯固有肌层)、Ⅲ期(肿瘤侵犯固有肌层并侵及浆膜下层/直肠系膜脂肪/内外括约肌间隙)和 Ⅳ期(肿瘤侵犯邻近脏器或结构)。
1.5 微血管生成判断
术中所收集的直肠癌组织和癌旁组织(距离肿瘤边缘>5 cm),经4%甲醛溶液固定后,石蜡包埋,并切成厚度约5 μm的切片,染色后测定MVD,将被CD34抗体免疫染色成孤立的棕黄色内皮细胞或内皮细胞团作为1个微血管[11],先在低倍镜(×40)下选择微血管数量最多的区域,随后转至高倍镜(×200)下,随机选择3个视野,所得均值即为MVD。
1.6 统计学处理
采用SPSS 21.0软件对数据进行统计学分析。计量资料以x±s表示,采用t检验或方差分析,多组比较后两两比较采用LSD-t法。计数资料以n (%)表示,采用χ2检验。采用Spearman相关系数分析直肠癌患者DCE-MRI参数与分化程度、临床分期及MVD的关系。P<0.05为差异有统计学意义。
2 结 果
2.1 对比癌组织、癌旁组织中的DCE-MRI定量分析参数和MVD
直肠癌组织的Kep值、Ktrans值、Ve值及MVD均高于癌旁组织(P<0.05,表1)。DCE-MRI图像和术后病理学检查结果见图1。
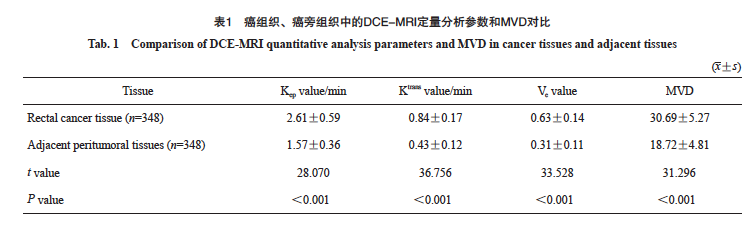
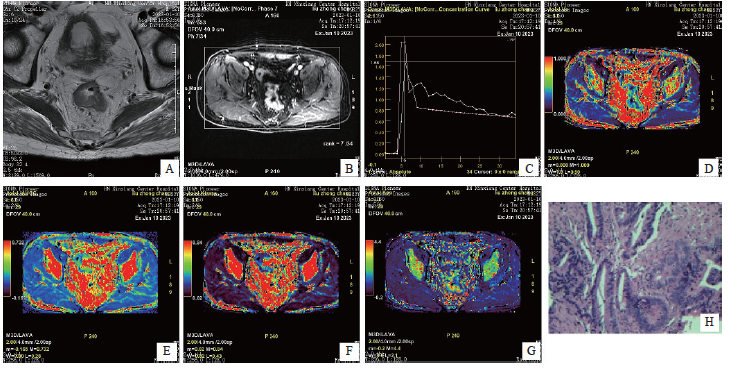
图1 DCE-MRI图像与术后病理学图像
Fig. 1 DCE-MRI image and postoperative pathology image
An 82-year-old male presented with anal distension and retraction for six months, and was subsequently diagnosed with rectal cancer by pathological examination. A: The intestinal wall in the middle and lower part of rectum is unevenly thickened, with slightly high signal on T2WI, and the lesion penetrates the muscularis propria; B: The lesion showed uneven and continuous enhancement; C: Time-signal intensity curve of DCE-MRI (pink is the lesion curve); D: Pseudo-color image, yellow-green area shows local occupation of intestinal wall; E, F: Pseudo-color image, red area shows local occupation of intestinal wall; G: Pseudo-color image, with red and yellow areas showing local occupation of intestinal wall; H: Postoperative pathological picture, diagnosed as rectal (H-E staining, ×200).
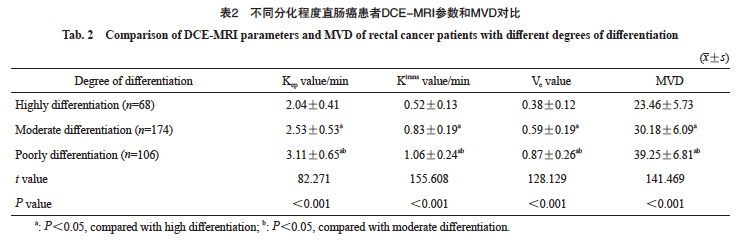
2.2 对比不同分化程度直肠癌患者的DCE-MRI定量分析参数和MVD
本研究的348例直肠癌患者中,高分化68例,中分化174例,低分化106例;不同分化程度直肠癌患者的Kep值、Ktrans值、Ve值及MVD对比,差异有统计学意义(P<0.05)。低分化和中分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于高分化直肠癌患者(P<0.05),低分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于中分化直肠癌患者(P<0.05,表2)。
2.3 对比不同分临床分期直肠癌患者的DCE-MRI定量分析参数和MVD
本研究的348例直肠癌患者中,Ⅰ期79例,Ⅱ期116例,Ⅲ期88例,Ⅳ期65例;不同分期直肠癌患者的Kep值、Ktrans值、Ve值及MVD对比,差异有统计学意义(P<0.05)。Ⅱ期、Ⅲ期和 Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅰ期直肠癌患者(P<0.05),Ⅲ期和Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅱ期直肠癌患者(P<0.05),Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅲ期直肠癌患者(P<0.05,表3)。

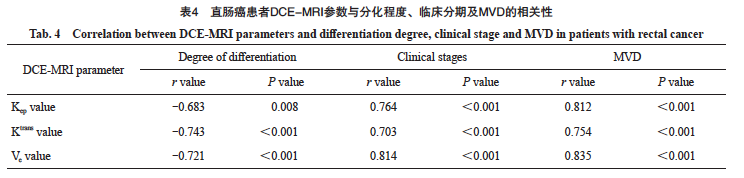
2.4 直肠癌患者的DCE-MRI参数与分化程度、临床分期及MVD的相关性
直肠癌患者的Kep值、Ktrans值、Ve值与分化程度均呈负相关(r=-0.683、-0.743、-0.721,P< 0.05),直肠癌患者的Kep值、Ktrans值及Ve值与临床分期均呈正相关(r=0.764、0.703、0.814,P<0.05),直肠癌患者的Kep值、Ktrans值及Ve值与MVD均呈正相关(r=0.812、0.754、0.835,P<0.05, 表4)。
3 讨 论
直肠癌是新生血管丰富的实体肿瘤,血管生成能力和转移能力较活跃[12-13]。新生血管的生成在直肠癌的增殖、侵袭及转移过程中发挥重要作用,新生血管不仅供给肿瘤快速生长所需的养分,同时还成为其转移的重要通道[14]。由于肿瘤血管生成是肿瘤生长、浸润及转移的基础,因此对肿瘤血管生成程度进行评估尤为重要。DCE-MRI为一种可反映肿瘤组织微循环结构和血管通透性的无创性功能成像技术,其主要通过测定对比剂经过组织器官所引起的T1WI信号强度变化获得病灶的血流灌注信息[15]。本研究通过探讨DCE-MRI参数与直肠癌微血管生成的关系,旨在为临床上该类患者的治疗及预后改善提供一定的指导依据。
本研究中,直肠癌组织的Kep值、Ktrans值、Ve值及MVD均高于癌旁组织,提示直肠癌组织DCE-MRI参数和MVD较高,大量血管生成和血管异常增强了对比剂的流入,分析原因可能是肿瘤血管与正常血管相比有所不同,如高血管通透性、不受控制的血管生成等[16],基于此,DCE-MRI对比剂在肿瘤与正常组织内的分布及代谢也存在差异。本研究结果显示,低分化和中分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于高分化直肠癌患者,低分化直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于中分化直肠癌患者,提示直肠癌患者的DCE-MRI参数、MVD与分化程度有关。分析原因可能是低分化肿瘤恶性程度较高,组织内不成熟新生血管较多,血流量增加,血管内皮因子对血管的刺激也更大,可增大血管的通透性[17];同时由于肿瘤细胞破坏血管或微血管成熟度较低使微血管壁完整较差,进而使对比剂更易透过血管壁外渗[18]。本研究结果显示,Ⅱ期、Ⅲ期和Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅰ期直肠癌患者,Ⅲ期和Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅱ期直肠癌患者,Ⅳ期直肠癌患者的Kep值、Ktrans值、Ve值及MVD均高于Ⅲ期直肠癌患者,提示直肠癌患者DCE-MRI参数、MVD与临床分期有关。分析原因可能是随着肿瘤分期越高,可促进微血管生成,导致MVD异常升高,并可增加血流量[19];此外,肿瘤分期越高,微血管的渗透性越好,进而可增大血管的通透性,致使DCE-MRI参数异常升高[20]。DCE-MRI通过分析ROI可获得对比剂吸收的定量参数,这些参数可反映血管解剖及生理学特征[21];Kep值表示单位时间由血管外细胞间隙渗入血管的对比剂量,Kep值越高,回流入血管的对比剂越多;Ktrans值是微血管通透性的定量参数,可反映肿瘤微血管的生长状态;Ve值为单位容积组织内细胞外血管外间隙的体积,Ve值越大,血管外细胞外间隙容积越大,说明组织细胞化程度越低、组织坏死程度越大。MVD是衡量血管生成活跃程度的定量指标,可反映血管的生成状态,随着MVD的升高,可增加肿瘤细胞转移、远处种植转移的风险[22]。Spearman相关系数分析结果显示,直肠癌患者的Kep值、Ktrans值及Ve值与分化程度呈负相关,直肠癌患者的Kep值、Ktrans值及Ve值与临床分期及MVD呈正相关。
综上所述,DCE-MRI参数与直肠癌分化程度、临床分期及微血管生成均相关,可为直肠癌患者术前评估提供更多有价值的影像学信息,具有潜在的临床应用价值。
第一作者兼通信作者:
宋丹,学士,主治医师。
作者贡献声明:
宋丹:研究设计和实施、数据分析及文章撰写;柴亚欣:研究实施、数据收集和分析;葛延平:数据分析、论文修改。
[参考文献]
[1] LYNCH P, RYAN O K, DONNELLY M, et al. Comparing neoadjuvant therapy followed by local excision to total mesorectal excision in the treatment of early stage rectal cancer: a systematic review and meta-analysis of randomised clinical trials[J]. Int J Colorectal Dis, 2023, 38(1): 263.
[2] FAROOQI M, HUSSAIN A, AHMAD A, et al. Impact of trans-anal versus laparoscopic total mesorectal excision on the surgical and pathologic outcomes of patients with rectal cancer: meta-analysis of randomized controlled trials[J]. Langenbecks Arch Surg, 2023, 408(1): 413.
[3] PERIVOLIOTIS K, NTELLAS P, DADOULI K, et al. Microvessel density (MVD) in patients with osteosarcoma: a systematic review and meta-analysis[J]. Cancer Invest, 2024, 42(1): 104-114.
[4] JIANG J J, LI J Z, XIONG X Y, et al. Different predictive values of microvessel density for biochemical recurrence among different PCa populations: a systematic review and metaanalysis[J]. Cancer Med, 2023, 12(3): 2166-2178.
[5] 柴亚欣, 牛永超. 动态对比增强MRI联合体素内不相干运动术前评估直肠癌病理分型[J]. 中国医学影像技术, 2023, 39(12): 1833-1837.
CHAI Y X, NIU Y C. Dynamic contrast enhanced MRI combined with intravoxel incoherent motion for preoperative evaluation on pathological type of rectal cancer[J]. Chin J Med Imag Technol, 2023, 39(12): 1833-1837.
[6] ARIAN A, SEYED-KOLBADI F Z, YAGHOOBPOOR S, et al. Diagnostic accuracy of intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced (DCE) MRI to differentiate benign from malignant breast lesions: a systematic review and metaanalysis[J]. Eur J Radiol, 2023, 167: 111051.
[7] 汪 洁, 包善磊, 胡 月, 等. 动态对比增强磁共振成像影像组学评估胶质瘤异柠檬酸脱氢酶1突变与微血管生成[J]. 放射学实践, 2023, 38(6): 685-692.
WANG J, BAO S L, HU Y, et al. Evaluation of IDH1 mutation and angiogenesis in gliomas by radiomics analysis based on DCE-MRI[J]. Radiol Pract, 2023, 38(6): 685-692.
[8] SHINAGAWA T, TANAKA T, NOZAWA H, et al. Comparison of the guidelines for colorectal cancer in Japan, the USA and Europe[J]. Ann Gastroenterol Surg, 2017, 2(1): 6-12.
[9] 李玉林. 病理学[M]. 7版. 北京: 人民卫生出版社, 2008: 79-80.
LI Y L. Pathology[M]. 7 Edition. Beijing: People’s Medical Publishing House, 2008: 79-80.
[10] TONG G J, ZHANG G Y, LIU J, et al. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: a retrospective review of our data[J]. World J Clin Oncol, 2018, 9(7): 148-161.
[11] WEIDNER N, FOLKMAN J, POZZA F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma[J]. J Natl Cancer Inst, 1992, 84(24): 1875-1887.
[12] PELTRINI R, IMPERATORE N, DI NUZZO M M, et al. Towards personalized treatment of T2N0 rectal cancer: a systematic review of long-term oncological outcomes of neoadjuvant therapy followed by local excision[J]. J Gastroenterol Hepatol, 2022, 37(8): 1426-1433.
[13] TAN S F, GAO Q Q, CUI Y P, et al. Oncologic outcomes of watch-and-wait strategy or surgery for low to intermediate rectal cancer in clinical complete remission after adjuvant chemotherapy: a systematic review and meta-analysis[J]. Int J Colorectal Dis, 2023, 38(1): 246.
[14] PERIVOLIOTIS K, SAMARA A A, KOUTOUKOGLOU P, et al. Microvessel density in differentiated thyroid carcinoma: a systematic review and meta-analysis[J]. World J Methodol, 2022, 12(5): 448-458.
[15] 牛 涛, 燕翠芳. DCE-MRI联合弥散加权成像对老年直肠癌诊断价值及术前分期评估[J]. 中国老年学杂志, 2024, 44(19): 4645-4647.
NIU T, YAN C F. Diagnostic value and preoperative staging evaluation of DCE-MRI combined with diffusion-weighted imaging in elderly rectal cancer[J]. Chin J Gerontol, 2024, 44(19): 4645-4647.
[16] ARIAN A, TAHER H J, SUHAIL NAJM ALAREER H, et al. Value of conventional MRI, DCE-MRI, and DWI-MRI in the discrimination of metastatic from non-metastatic lymph nodes in rectal cancer: a systematic review and meta-analysis study[J]. Asian Pac J Cancer Prev, 2023, 24(2): 401-410.
[17] 吕 霞, 刘 强, 刘 岘, 等. 直肠癌DCE-MRI定量参数与病理分化程度和p53的相关性[J]. 实用医学杂志, 2022, 38(4): 479-483.
LÜ X, LIU Q, LIU X, et al. An analysis on association of DCEMRI quantitative parameters with pathological differentiation and p53 in rectal cancer[J]. J Pract Med, 2022, 38(4): 479-483.
[18] MULYADI R, PUTRI P P, HANDOKO H, et al. Dynamic contrast-enhanced magnetic resonance imaging parameter changes as an early biomarker of tumor responses following radiation therapy in patients with spinal metastases: a systematic review[J]. Radiat Oncol J, 2023, 41(4): 225-236.
[19] 杨彦松, 李 君, 张明珠, 等. DCE-MRI定量参数联合高分辨率T2WI预测直肠腺癌病理N分期的价值[J]. 放射学实践, 2023, 38(4): 459-467.
YANG Y S, LI J, ZHANG M Z, et al. Value of quantitative parameters of dynamic contrast-enhanced MRI combined with high-resolution T2WI findings in predicting pathological N-stage of rectal adenocarcinoma[J]. Radiol Pract, 2023, 38(4): 459-467.
[20] ZHANG J, WANG L, LIU H F. Imaging features derived from dynamic contrast-enhanced magnetic resonance imaging to differentiate malignant from benign breast lesions: a systematic review and meta-analysis[J]. J Comput Assist Tomogr, 2022, 46(3): 383-391.
[21] SHOMAL ZADEH F, POOYAN A, ALIPOUR E, et al. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in differentiation of soft tissue sarcoma from benign lesions: a systematic review of literature[J]. Skeletal Radiol, 2024, 53(7): 1343-1357.
[22] 杨 炼, 肖 明, 李 娴, 等. 人结直肠癌组织及LOVO细胞中ARTC1的表达及其与肿瘤微血管生成的相关性[J]. 临床与实验病理学杂志, 2022, 38(10): 1198-1203.
YANG L, XIAO M, LI X, et al. Expression of ARTC1 in human colorectal cancer tissues and LOVO cells and its relationship with microangiogenesis[J]. Chin J Clin Exp Pathol, 2022, 38(10): 1198-1203.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)