首页 > 医疗资讯/ 正文
1 传统药物
当前,传统药物在PsA治疗中仍占据重要地位,多数指南认为,传统合成改善病情抗风湿药物(csDMARDs)可有效控制或延缓PsA病情进展[1-2],包括甲氨蝶呤、来氟米特和柳氮磺吡啶。
值得注意的是,区别于新研发的生物制剂,csDMARDs在PsA中的疗效缺乏来自大型随机双盲安慰剂对照研究的数据[3],且仅有的一项针对甲氨蝶呤的随机对照试验未显示甲氨蝶呤具有优于安慰剂的疗效[4]。
但在临床和治疗策略研究中,甲氨蝶呤显示出对包括PsA综合疾病活动度指标、指炎、附着点炎、皮损、关节肿痛计数等在内的多方位疗效[5-7]。与甲氨蝶呤类似,来氟米特、柳氮磺吡啶对PsA外周关节受累也具有一定治疗作用,但在皮损的治疗中甲氨蝶呤具有更好的疗效[8-9]。结合更低的药物费用和临床可及性,csDMARDs在PsA患者中广泛使用并被指南推荐作为PsA一线治疗药物。
在众多csDMARDs药物中,指南除提及在合并PsA相关皮肤受累的患者中首选甲氨蝶呤外,对csDMARDs类药物选择并未给出优先顺序。一项研究比较了真实世界中不同单药方案治疗PsA的药物留存率,为间接评估csDMARDs疗效提供了线索,结果显示甲氨蝶呤的药物留存率显著高于柳氮磺吡啶,这一结果为临床实践中药物选择提供了一定指导[10]。
除既往研究中涉及的csDMARDs单药的有效性外,一项随机对照研究评估了甲氨蝶呤联合来氟米特治疗PsA的疗效是否优于甲氨蝶呤单药治疗[11],数据显示虽然联合治疗可更大程度上改善PsA患者的疾病活动度[银屑病关节炎活动度评分(PASDAS)治疗差异为-0.6, 90% CI:-1.0~-0.1,P=0.025],但联合治疗的耐受性不如单药治疗,其轻度不良事件发生率更高,基于该结果指南未推荐csDMARDs联合疗法。
此外,甲氨蝶呤联合生物制剂能否提升生物制剂治疗PsA的有效性亦是临床关注的焦点。近期一项纳入31篇研究的荟萃分析显示[12],与生物制剂单药治疗相比,甲氨蝶呤联合生物制剂在美国风湿病协会(ACR)20应答率(HR=0.97,95% CI:0.85~1.11)和ACR50应答率(HR=0.96,95% CI:0.81~1.14)方面均无显著优势,这提示甲氨蝶呤联合治疗的意义可能有限。
2 生物制剂
生物制剂亦称生物类改善病情抗风湿药物(bDMARDs),其已成为PsA治疗的重要组成部分。根据作用机制的不同,用于PsA治疗的bDMARDs可分为4大类:即肿瘤坏死因子抑制剂(TNFi)、白细胞介素(IL)-12/23抑制剂和IL-23抑制剂、IL-17抑制剂、细胞毒性T淋巴细胞相关抗原4(CTLA4)激动剂。根据最近欧洲抗风湿病联盟(EULAR)的建议,对于外周关节炎患者,如果对至少一种csDMARDs反应不足,则应开始使用bDMARDs治疗[1]。
此外,治疗药物选择还应将PsA相关的非肌肉骨骼症状纳入考量,比如临床出现了明显的皮肤受累,则首选IL-17抑制剂或IL-12/23抑制剂和IL-23抑制剂;而对于合并葡萄膜炎的PsA患者,则首选TNFi。
2.1 TNFi
肿瘤坏死因子(TNF)是细胞因子成员,其可通过影响细胞过程而调节炎症反应和免疫功能,在PsA的发病机制中起重要作用[13]。研究发现,TNF在PsA患者的皮肤和滑膜中均升高,因此被认为是一个重要的治疗靶点,且TNFi是首批获准用于治疗PsA的生物制剂。既往研究表明,TNFi可阻止PsA关节的影像学进展,延缓骨侵蚀[14-17]。目前已有5种已获批的TNFi,包括阿达木单抗、依那西普、英夫利昔单抗、培塞利珠单抗和戈利木单抗[18]。
大量研究已证实,这些不同类型的TNFi在控制PsA炎性症状、抑制外周关节结构损伤进展、改善患者生活质量和身体功能方面具有显著作用[17,19-23],且其疗效不仅针对外周关节受累,对指炎、附着点炎、皮损均显示出良好疗效[24-26]。不同TNFi在抗体结构、用法存在各自特点(表1),临床应结合患者自身特点选择不同的TNFi,如妊娠期和哺乳期女性更适宜采用培塞利珠单抗。
表1 不同TNFi的特点
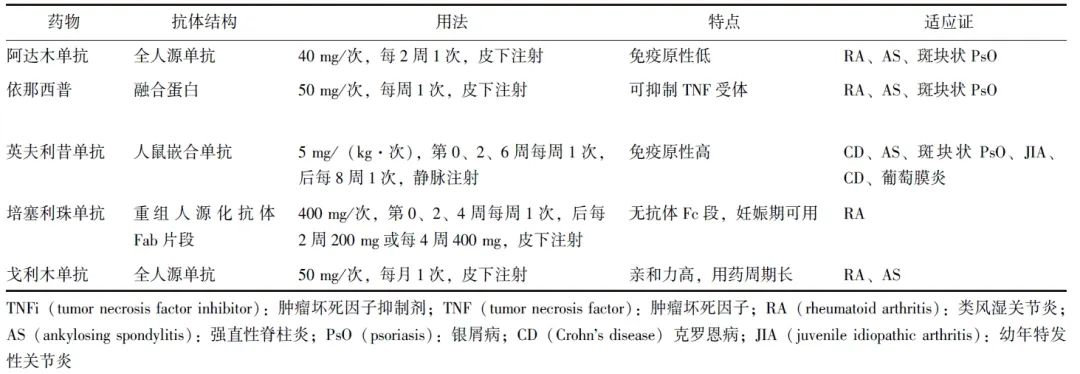
在疗效明确的背景下,更多研究者进一步聚焦于影响TNFi有效性的因素。血药浓度与药物疗效密切相关,一项研究评价了阿达木单抗和依那西普血药浓度与治疗3个月时临床疗效的相关性[27],研究纳入了97例使用依那西普和104例使用阿达木单抗治疗的PsA患者,结果显示3个月达到治疗应答患者的血药浓度更高。该研究分别给出了此2种药物对应的药物浓度阈值,为在临床实践中优化治疗效果提供了参考。
另一项近期开展的研究评估了性别对首次接受TNFi治疗的PsA患者治疗应答和药物留存率的影响[28]。该研究分析了来自欧洲脊柱关节炎研究协作网络注册中心的7679例PsA患者(女性占比49.6%)的数据,结果显示在基线疾病特征相似的情况下,女性PsA患者接受首次TNFi治疗的应答率和药物留存率均更低,治疗6个月时64%的女性和78%的男性达到了低疾病活动度(RR=0.82,95% CI:0.80~0.84)。男性患者在用药6个月、12个月和24个月的药物留存率分别为88%、77%和64%,女性患者中分别为79%、64%和50%,差异具有统计学意义(P均<0.001),这强调了在PsA临床管理和药物治疗中考虑性别差异的重要性。
2.2 IL-12/23抑制剂和IL-23抑制剂
近年来,针对PsA发病机制的探索取得了显著性进展,其中对于IL-12/23与IL-17细胞因子轴作为主要驱动因素的认识尤为关键[29-30]。这一重大发现极大提升了科研人员对研发直接针对该通路治疗方法的热情,尤其是阻断IL-17或IL-12/IL-23的靶向药物。
2.2.1 乌司奴单抗(Ustekinumab)
乌司奴单抗是一种全人源化靶向IL-12和IL-23共有的p40亚基的IgG1k单克隆抗体。两项Ⅲ期临床对照试验PSUMMIT 1和PSUMMIT 2证实了乌司奴单抗在未使用过生物制剂及先前对TNFi治疗反应不足PsA患者中的疗效[31-32],并使得该药先后在美国、欧洲上市,其于2019年在我国获批用于治疗对其他系统治疗无应答、有禁忌或无法耐受的成年中重度斑块状银屑病患者。
PsABio研究是一项针对PsA患者的跨国前瞻性观察性研究[33],该项为期3年的研究提供了长期数据,旨在评估乌司奴单抗和TNFi作为PsA患者一线至三线治疗处方的持久性、有效性和安全性。结果显示,在随访3年时,乌司奴单抗与TNFi治疗的药物留存率相当(49.9% 比47.8%),但乌司奴单抗组不良事件发生率更低(34.6%比39.7%)。该研究验证了乌司奴单抗的疗效及其安全性,并指出皮肤受损伤程度高且甲氨蝶呤使用禁忌的PsA患者更适合使用乌司奴单抗治疗而非TNFi。
2.2.2 古塞奇尤单抗(Guselkumab)
古塞奇尤单抗是一种针对IL-23的p19亚基而研发的全人源IgG1单克隆抗体,在安慰剂随机对照DISCOVER-1和DISCOVER-2 Ⅲ期试验中该药治疗PsA的疗效和安全性得到了认可[34-35]。与安慰剂组相比,古塞奇尤单抗组治疗24周时达到主要终点ACR20应答的患者比例明显更高(DISCOVER-1:59%比22%, P<0.0001;DISCOVER-2:64%比33%, P<0.0001)且疗效可持续至用药52周[36-37]。
另一项研究基于此两项Ⅲ期临床试验的事后分析[38],结果发现古塞奇尤单抗可使更多患者在早期(治疗4周或8周)即可达到最小有临床意义的改善(MCII),且早期达到MCII者在24周和52周时更可能实现长期疾病控制。值得注意的是,在治疗早期未达MCII的患者中,仍有相当比例的患者在52周时实现了更为严格的疾病控制。因此,早期达到MCII并非是PsA患者长期预后唯一的决定因素,但其可为患者的疾病管理提供重要指引。
2.2.3 利生奇珠单抗(Risankizumab)
利生奇珠单抗是另一种针对IL-23 p19亚基的人源化IgG1单克隆抗体,通过阻断IL-23与其受体的结合,可抑制IL-23依赖性细胞间信号传导和促炎因子释放,从而发挥治疗PsA的作用。2022年两项随机双盲Ⅲ期试验KEEPsAKE 1和KEEPsAKE 2结果公布,证明利生奇珠单抗可显著改善PsA患者的关节和皮肤症状,患者耐受性良好[39-40],且后续52周的数据进一步证实该药治疗PsA的长期有效性与安全性[41-42]。
需说明的是,在非甾体抗炎药治疗反应不足的中轴受累PsA患者中推荐使用TNFi、IL-17抑制剂和JAK抑制剂,而非IL-12/23抑制剂和IL-23抑制剂。这是因为虽然IL-12/23抑制剂乌司奴单抗[43]和古塞奇尤单抗[44]临床研究的事后分析证实其在强直性脊柱炎疾病活动度指数(BASDAI)和强直性脊柱炎疾病活动评分(ASDAS)方面具有改善作用,但这些药物未被证明对中轴型脊柱关节炎有效,且目前相关证据有限且存在相互矛盾之处,故指南尚未推荐其治疗PsA的中轴病变。
2.3 IL-17抑制剂
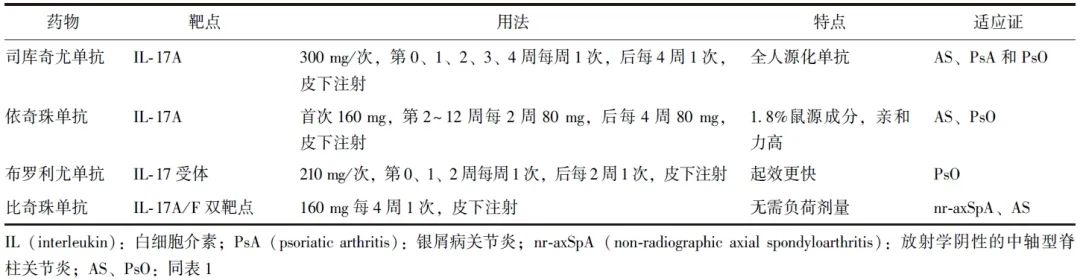
来自IL-17家族的细胞因子在炎症反应中亦发挥关键作用。虽然最初IL-17细胞因子被认为具有抵抗细菌和真菌感染的保护功能,但后续研究发现IL-17细胞因子可促进包括PsA在内的多种免疫性疾病的慢性炎症进展[45]。目前美国食品药品监督管理局已批准4种IL-17抑制剂用于治疗PsA(表2)。
表2 不同IL-17抑制剂的特点
2.3.1 司库奇尤单抗(Secukinumab)
司库奇尤单抗为针对IgG1的全人源单克隆抗体,其作用机制是通过直接抑制IL-17A而发挥作用。美国开展的一项临床研究显示,司库奇尤单抗可改善PsA患者的疾病活动度和生活质量[46]。在真实世界研究中,该药物还能显著减轻疼痛、趾炎、附着点炎及皮肤和指甲受累[47]。
来自FUTURE5临床实验的2年随访数据证实了司库奇尤单抗在PsA患者中展示出持续的临床疗效和低影像学进展的疗效。该研究将996例PsA患者随机分为不同剂量的治疗组后,783例(78.6%)完成了2年治疗。结果显示,临床终点的改善在2年内持续存在;治疗2年时,不同剂量治疗组别无影像学进展的患者比例均超过80%[48]。
一项基于真实世界数据的回顾性、多中心、观察性队列研究[49]纳入了来自SUPREME研究的297例中重度慢性斑块状银屑病患者,数据显示司库奇尤单抗的长期有效性和安全性均满意,210例(70.7%)可在42个月的观察期内维持司库奇尤单抗治疗;随访42个月后,79.6%的患者达到银屑病面积和严重程度指数(PASI)90应答。
2.3.2 依奇珠单抗(Ixekizumab)
依奇珠单抗是另一种可抑制IL-17A的人源单克隆抗体,对皮肤、关节症状亦显示出良好疗效[50-51]。一项纳入1401例PsA患者且依奇珠单抗暴露量超过2000患者年的研究证明了该药物的长期安全性,数据显示调整后的不良事件发生率为50.3/100患者年,且多数不良事件为轻中度[52]。与司库奇尤单抗相比,依奇珠单抗的10年药物留存率更高(50%比40%)[53],上述研究提示依奇珠单抗或可具有更长期的疗效和更高的安全性,但确切结论需更多数据予以支持。
2.3.3 布罗利尤单抗(Brodalumab)
布罗利尤单抗是一种靶向IL-17受体的全人源IgG2单抗,可同时阻断IL-17家族众多亚型之间的信号传导[54]。Ⅲ期临床试验显示,与安慰剂相比,布罗利尤单抗可快速、显著改善PsA患者的体征和症状;且安全性与其他IL-17抑制剂相当[55]。
一项系统综述显示,相较于其他IL-17抑制剂,布罗利尤单抗的起效时间更早,有助于快速控制临床症状[56]。Kojanova等[57]在研究中亦证明了布罗利尤单抗的优势,与依奇珠单抗和司库奇尤单抗相比,该药治疗3个月后PASI75、PASI90和PASI100应答率最高(布罗利尤单抗:91.7%、76.4%和58.7%;依奇珠单抗:88.3%、71.3%和49.2%;司库奇尤单抗:84.0%、60.7%和38.6%)。
2.3.4 比奇珠单抗(Bimekizumab)
IL-17A和IL-17F在病理性骨形成中协同作用,同时抑制此2种炎症因子比单独抑制IL-17A可能具有更好的疗效。比奇珠单抗是一款IL-17A/F抑制剂,在一项纳入206例患者的Ⅱb期临床试验(BEACTIVE)中,比奇珠单抗在16 mg、160 mg(无负荷剂量组)和160 mg(有负荷剂量组)中均显示出良好的疗效和安全性,且起效迅速。
与安慰剂组相比,16 mg组(OR=4.2,95% CI:1.1~15.2,P=0.032)、160 mg(无负荷剂量组)(OR=8.1,95% CI:2.3~28.7,P=0.0012)和160 mg(有负荷剂量组)(OR=9.7,95% CI:2.7~34.3,P=0.0004)ACR50应答率显著增加,且疗效可维持至用药48周[58]。此外,对于既往未曾使用过生物制剂或对TNFi不耐受/反应差的患者,比奇珠单抗也被认为是比司库奇尤单抗更有效的替代方案[59]。
2.4 CTLA4激动剂
阿巴西普(Abatacept)是一种可溶性融合蛋白,可与CTLA-4的细胞外结构域和人类IgG1修饰的Fc片段部分融合。Ⅱ期和Ⅲ期临床试验显示,与安慰剂相比,静脉注射和皮下注射阿巴西普均可获得更高的ACR20应答率[60-61]和经MRI影像学证实的更满意的滑膜炎和腱鞘炎改善程度[62]。
3 靶向合成改善病情抗风湿药物
3.1 JAK抑制剂
Janus激酶抑制剂(JAKi)是可阻断JAK激酶活性,从而下调在PsA发病中起重要作用的细胞因子产生的一类小分子靶向药物,目前研究较多的为托法替布(Tofacitinib)、乌帕替尼(Upadacitinib)和非戈替尼(Filgotinib)。
托法替布为泛JAK抑制剂,主要针对JAK1和JAK3起治疗功效,而对JAK2的抑制作用较弱,两项高质量Ⅲ期临床试验证实了托法替布对控制PsA疾病活动度的良好疗效[63],目前其已被中国国家药品监督管理局批准用于对一种或多种csDMARDs应答不佳或不耐受的活动性PsA成人患者。
乌帕替尼是一种更具选择性的JAK抑制剂,主要通过抑制JAK1的活性而发挥作用,SELECT-PsA 1 和 SELECT-PsA 2 Ⅲ期临床试验显示,其可改善PsA疾病活动度,且疗效优于阿达木单抗[64-66];进一步对上述临床试验进行扩展分析后发现,乌帕替尼的疗效可持续至用药第56周且安全性数据与先前报道一致。
非戈替尼也是一种选择性口服JAK1抑制剂,Ⅱ期临床试验(EQUATOR)对其在PsA中的疗效进行了评估[67]。该研究共纳入131例csDMARDs未能控制疾病活动度的PsA患者,结果发现非戈替尼起效迅速,治疗第1周即可观察到治疗反应,在治疗16周时ACR20应答率显著高于安慰剂组(80%比33%,P<0.0001)。既往研究曾指出非戈替尼具有潜在的睾丸毒性,而MANTA和MANTA-RAy临床试验证实,每日1次的非戈替尼200 mg持续13周,未发现对患有活动性炎症性肠病和炎性关节炎男性患者的精液参数或性激素产生可检测的影响[68]。
最新发表的一项荟萃分析对JAK抑制剂的安全性和有效性进行了系统分析,该研究纳入5项随机对照试验(2项针对托法替尼的Ⅲ期研究、1项针对非戈替尼的Ⅱ期研究和2项针对乌帕替尼的Ⅲ期研究)共3293例接受不同JAK抑制剂治疗的PsA患者,结果表明JAK抑制剂的疗效方面显著优于安慰剂(结局指标:ACR20应答率,OR=3.78, 95% CI:2.72~5.24),且无新的不良事件发生[69]。
但值得注意的是,一项2022年的随机、开放标签、非劣效研究纳入了存在至少1项心血管危险因素且年龄50岁以上的类风湿关节炎(RA)患者,并将其随机分配至托法替布组和TNFi组[70]。结果显示在主要心血管不良事件(HR=1.33, 95% CI:0.91~1.94)和恶性肿瘤(HR=1.48, 95% CI:1.04~2.09)发生率方面,托法替布组显著高于TNFi组(显著性定义:95% CI上限大于1.8)。对数据进行事后分析显示,除托法替布外,其他增加主要心血管不良事件的危险因素包括年龄≥65岁、高基线心血管风险、吸烟史、持续炎症等[71-74]。此外托法替布组比TNFi组具有更高的血栓发生风险[75]。这提示在具有心血管危险因素的老年患者中使用托法替布可能需更加谨慎。
3.2 TYK抑制剂
酪氨酸激酶2(TYK2)是一种细胞内激酶,通过IL-23、IL-12和I型干扰素介导PsA炎症反应发生。不同于JAK家族其他成员(JAK1、JAK2和JAK3参与免疫通路及更广泛的免疫外通路如骨髓造血、脂质代谢等),TYK2信号通路局限于免疫通路,作用位点更具特异性。因此TYK2抑制剂在阻断TYK2信号传导发挥治疗作用的同时表现出更好的安全性。
3.2.1 氘可来昔替尼(Deucravacitinib)
氘可来昔替尼是一种新型口服选择性TYK2抑制剂,在PsA治疗中具有良好潜力。Ⅱ期临床试验数据表明[76],与安慰剂相比(31.8%),6 mg/d组(52.9%)和12 mg/d组(62.7%)氘可来昔替尼在第16周时均具有更高的ACR20应答率(主要结局指标),并在多项次要结局指标(包括ACR50/70应答率和附着点炎)中亦展现出更好的疗效,且随访期间未发现静脉血栓栓塞或血液学异常相关安全问题。
3.2.2 Brepocitinib
Brepocitinib是TYK2和JAK1的小分子抑制剂,一项为期52 周随机安慰剂对照Ⅱb期临床试验对其应用价值进行了评估[77]。该研究将218名受试者随机分配至安慰剂组和不同剂量的Brepocitinib组。疗效分析显示,在第16周时,30 mg/d Brepocitinib组(66.7%)和60 mg/d Brepocitinib组(74.6%)ACR20响应率显著优于安慰剂组(43.3%),且ACR50、ACR70、PASI75、PASI90、最小疾病活动(MDA)等指标的应答率明显更高,其药物疗效持续可至用药第52周,该研究展示了Brepocitinib在PsA中强力的临床疗效及作为未来治疗药物的潜力。
4 小结与展望
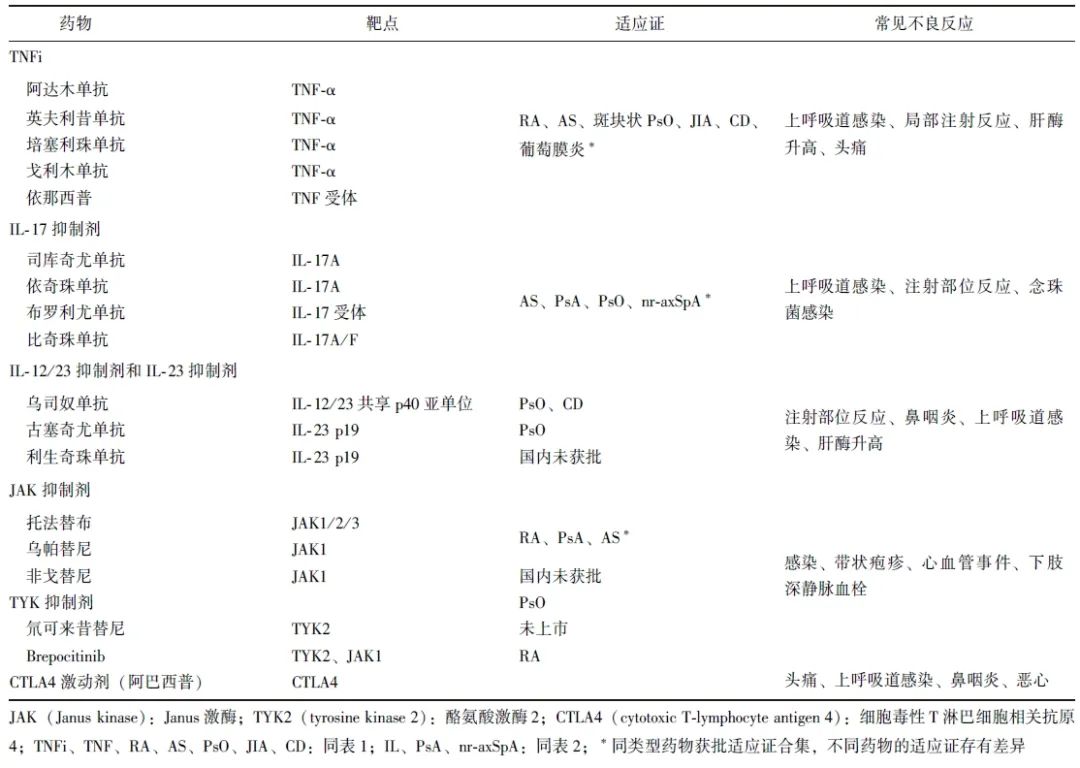
作为一种慢性炎症性疾病,PsA可累及脊柱、关节、四肢,病情迁延难愈,易复发,目前临床治疗以对症治疗,控制症状为主。笔者总结了各类药物靶点、适应证及常见不良反应(表3)。
表3 生物和靶向合成改善病情抗风湿药物的靶点、适应证及常见不良事件
csDMARDs在PsA的药物治疗中仍扮演着关键角色,并因疗效确切得到了临床指南的推荐。然而,其临床应用过程中亦面临诸多挑战,如起效慢、可引起严重副作用(如肝肾损伤)。
bDMARDs可针对不同靶点发挥治疗作用,安全性得到了提升,为PsA患者提供了新的治疗选择,但其价格高昂、给药方式复杂(如皮下注射或静脉注射)一定程度上限制了其临床推广。
新型JAK抑制剂作为一种小分子靶向药物,具有口服给药的便利性和显著疗效,但需警惕可引起血脂异常和血栓形成等不良反应的风险。
在临床实践中,医生需与患者共同讨论不同药物的风险、治疗目标和费用,结合患者自身特点及合并症情况,选取最适宜的药物,实现医患共同决策。相信随着对PsA发病机制的深入研究,未来将有更多新型药物和治疗策略涌现,从而为PsA患者提供更加全面、高效、精准的治疗方案。
参考文献
[1]Gossec L, Kerschbaumer A, Ferreira R J O, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update[J]. Ann Rheum Dis, 2024, 83(6): 706-719.
[2]Singh J A, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis[J]. Arthritis Care Res (Hoboken), 2019, 71(1): 2-29.
[3]Maese J, Díaz Del Campo P, Seoane-Mato D, et al. Effectiveness of conventional disease-modifying antirheumatic drugs in psoriatic arthritis: a systematic review[J]. Reumatol Clin (Engl Ed), 2018, 14(2): 81-89.
[4]Kingsley G H, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis[J]. Rheumatology (Oxford), 2012, 51(8): 1368-1377.
[5]Coates L C, Helliwell P S. Methotrexate efficacy in the tight control in psoriatic arthritis study[J]. J Rheumatol, 2016, 43(2): 356-361.
[6]Mease P J, Gladman D D, Collier D H, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase Ⅲ trial[J]. Arthritis Rheumatol, 2019, 71(7): 1112-1124.
[7]van Mens L J J, de Jong H M, Fluri I, et al. Achieving remission in psoriatic arthritis by early initiation of TNF inhibition: a double-blind, randomised, placebo-controlled trial of golimumab plus methotrexate versus placebo plus methotrexate[J]. Ann Rheum Dis, 2019, 78(5): 610-616.
[8]Clegg D O, Reda D J, Mejias E, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A department of veterans affairs cooperative study[J]. Arthritis Rheum, 1996, 39(12): 2013-2020.
[9]Kaltwasser J P, Nash P, Gladman D, et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial[J]. Arthritis Rheum, 2004, 50(6): 1939-1950.
[10]Jacobs M E, Pouw J N, Welsing P, et al. First-line csDMARD monotherapy drug retention in psoriatic arthritis: methotrexate outperforms sulfasalazine[J]. Rheumatology (Oxford), 2021, 60(2): 780-784.
[11]Mulder M L M, Vriezekolk J E, Van Hal T W, et al. Comparing methotrexate monotherapy with methotrexate plus leflunomide combination therapy in psoriatic arthritis (COMPLETE-PsA): a double-blind, placebo-controlled, randomised, trial[J]. Lancet Rheumatol, 2022, 4(4): e252-e261.
[12]Mease P J, Reddy S, Ross S, et al. Evaluating the efficacy of biologics with and without methotrexate in the treatment of psoriatic arthritis: a network meta-analysis[J]. RMD Open, 2024, 10(1): e003423.
[13]D'Angelo S, Cantini F, Ramonda R, et al. Effectiveness of adalimumab for the treatment of psoriatic arthritis: an Italian real-life retrospective study[J]. Front Pharmacol, 2019, 10: 1497.
[14]Mease P J, Gladman D D, Ritchlin C T, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial[J]. Arthritis Rheum, 2005, 52(10): 3279-3289.
[15]Mease P J, Kivitz A J, Burch F X, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression[J]. Arthritis Rheum, 2004, 50(7): 2264-2272.
[16]Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study[J]. Arthritis Rheum, 2009, 60(4): 976-986.
[17]Mease P J, Fleischmann R, Deodhar A A, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA)[J]. Ann Rheum Dis, 2014, 73(1): 48-55.
[18]Sundanum S, Orr C, Veale D. Targeted therapies in psoriatic arthritis-an update[J]. Int J Mol Sci, 2023, 24(7): 6384.
[19]Burmester G R, Panaccione R, Gordon K B, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease[J]. Ann Rheum Dis, 2013, 72(4): 517-524.
[20]Antoni C, Krueger G G, De Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial[J]. Ann Rheum Dis, 2005, 64(8): 1150-1157.
[21]Chimenti M S, Conigliaro P, Caso F, et al. Long-term effectiveness and drug survival of golimumab in patients affected by psoriatic arthritis with cutaneous involvement[J]. Clin Rheumatol, 2022, 41(1): 75-84.
[22]Kavanaugh A, McInnes I B, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study)[J]. Ann Rheum Dis, 2014, 73(9): 1689-1694.
[23]Esposito M, Carubbi F, Giunta A, et al. Certolizumab pegol for the treatment of psoriatic arthritis and plaque psoriasis[J]. Expert Rev Clin Immunol, 2020, 16(2): 119-128.
[24]Baranauskaite A, Raffayová H, Kungurov N V, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study[J]. Ann Rheum Dis, 2012, 71(4): 541-548.
[25]Kavanaugh A, Husni M E, Harrison D D, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study[J]. Arthritis Rheumatol, 2017, 69(11): 2151-2161.
[26]Mease P, Deodhar A, Fleischmann R, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure[J]. RMD Open, 2015, 1(1): e000119.
[27]Curry P D K, Hum R M, Morris A P, et al. Non-trough serum drug levels of adalimumab and etanercept are associated with response in patients with psoriatic arthritis[J/OL]. Rheumatology (Oxford): kead666.[2024-10-15]. https://doi.org/10.1093/rheumatology/kead666.
[28]Hellamand P, Van De Sande M G H, rnbjerg L M, et al. Sex differences in the effectiveness of first-line tumor necrosis factor inhibitors in psoriatic arthritis: results from the European spondyloarthritis research collaboration network[J]. Arthritis Rheumatol, 2024, 76(4): 587-598.
[29]Iwakura Y, Nakae S, Saijo S, et al. The roles of IL-17A in inflammatory immune responses and host defense against pathogens[J]. Immunol Rev, 2008, 226: 57-79.
[30]Miossec P, Korn T, Kuchroo V K. Interleukin-17 and type 17 helper T cells[J]. N Engl J Med, 2009, 361(9): 888-898.
[31]McInnes I B, Kavanaugh A, Gottlieb A B, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial[J]. Lancet, 2013, 382(9894): 780-789.
[32]Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial[J]. Ann Rheum Dis, 2014, 73(6): 990-999.
[33]Gossec L, Siebert S, Bergmans P, et al. Long-term effectiveness and persistence of ustekinumab and TNF inhibitors in patients with psoriatic arthritis: final 3-year results from the PsABio real-world study[J]. Ann Rheum Dis, 2023, 82(4): 496-506.
[34]Deodhar A, Helliwell P S, Boehncke W H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10230): 1115-1125.
[35]Mease P J, Rahman P, Gottlieb A B, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10230): 1126-1136.
[36]Ritchlin C T, Helliwell P S, Boehncke W H, et al. Guselkumab, an inhibitor of the IL-23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase Ⅲ randomised study of patients who were biologic-nave or TNFα inhibitor-experienced[J]. RMD Open, 2021, 7(1): e001457.
[37]McInnes I B, Rahman P, Gottlieb A B, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis[J]. Arthritis Rheumatol, 2021, 73(4): 604-616.
[38]Curtis J R, Deodhar A, Soriano E R, et al. Early improvements with guselkumab associate with sustained control of psoriatic arthritis: post hoc analyses of two phase 3 trials[J]. Rheumatol Ther, 2024, 11(6): 1501-1517.
[39]Kristensen L E, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial[J]. Ann Rheum Dis, 2022, 81(2): 225-231.
[40]Östör A, Van Den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial[J]. Ann Rheum Dis, 2022, 81(3): 351-358.
[41]Kristensen L E, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study[J]. Rheumatology (Oxford), 2023, 62(6): 2113-2121.
[42]Östör A, Van Den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 2 study[J]. Rheumatology (Oxford), 2023, 62(6): 2122-2129.
[43]Kavanaugh A, Puig L, Gottlieb A B, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase Ⅲ, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2)[J]. Ann Rheum Dis, 2016, 75(11): 1984-1988.
[44]Helliwell P S, Gladman D D, Chakravarty S D, et al. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-nave active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials[J]. RMD Open, 2020, 6(1): e001149.
[45]Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis[J]. Clin Rev Allergy Immunol, 2018, 55(3): 379-390.
[46]Kivitz A J, Kremer J M, Legerton C W 3rd, et al. Efficacy and safety of secukinumab in US patients with psoriatic arthritis: a subgroup analysis of the phase 3 FUTURE studies[J]. Rheumatol Ther, 2024, 11(3): 675-689.
[47]Alegre-Sancho J J, Núez-Monje V, Campos-Fernández C, et al. Real-world effectiveness and persistence of secukinu-mab in the treatment of patients with psoriatic arthritis[J]. Front Med (Lausanne), 2023, 10: 1294247.
[48]Mease P J, Landewé R, Rahman P, et al. Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year (end-of-study) results from the FUTURE 5 study[J]. RMD Open, 2021, 7(2): e001600.
[49]Russo F, Galluzzo M, Stingeni L, et al. Long-term drug survival and effectiveness of secukinumab in patients with moderate to severe chronic plaque psoriasis: 42-month results from the SUPREME 2.0 study[J]. Clin Cosmet Investig Dermatol, 2023, 16: 3561-3574.
[50]Gladman D D, Orbai A M, Klitz U, et al. Ixekizumab and complete resolution of enthesitis and dactylitis: integrated analysis of two phase 3 randomized trials in psoriatic arthritis[J]. Arthritis Res Ther, 2019, 21(1): 38.
[51]Lebwohl M G, Gordon K B, Gallo G, et al. Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER-3 study[J]. J Eur Acad Dermatol Venereol, 2020, 34(2): 301-309.
[52]Deodhar A A, Combe B, Accioly A P, et al. Safety of ixekizumab in patients with psoriatic arthritis: data from four clinical trials with over 2000 patient-years of exposure[J]. Ann Rheum Dis, 2022, 81(7): 944-950.
[53]Takami K, Tsuji S, Sato S, et al. Long-term retention rates of anti-tumour necrosis factor and anti-interleukin-17 antibodies for patients with psoriatic arthritis[J]. Mod Rheumatol, 2024, 34(5): 1013-1018.
[54]Facheris P, Valenti M, Pavia G, et al. Brodalumab: a new way to inhibit IL-17 in psoriasis[J]. Dermatol Ther, 2020, 33(3): e13403.
[55]Mease P J, Helliwell P S, Hjuler K F, et al. Brodalumab in psoriatic arthritis: results from the randomised phase Ⅲ AMVISION-1 and AMVISION-2 trials[J]. Ann Rheum Dis, 2021, 80(2): 185-193.
[56]Egeberg A, Andersen Y M F, Halling-Overgaard A S, et al. Systematic review on rapidity of onset of action for interleukin-17 and interleukin-23 inhibitors for psoriasis[J]. J Eur Acad Dermatol Venereol, 2020, 34(1): 39-46.
[57]Kojanova M, Hugo J, Velackova B, et al. Efficacy, safety, and drug survival of patients with psoriasis treated with IL-17 inhibitors - brodalumab, ixekizumab, and secukinumab: real-world data from the Czech Republic BIOREP registry[J]. J Dermatolog Treat, 2022, 33(6): 2827-2837.
[58]Ritchlin C T, Kavanaugh A, Merola J F, et al. Bimekizu-mab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial[J]. Lancet, 2020, 395(10222): 427-440.
[59]Mease P J, Warren R B, Nash P, et al. Comparative effectiveness of bimekizumab and secukinumab in patients with psoriatic arthritis at 52 weeks using a matching-adjusted indirect comparison[J]. Rheumatol Ther, 2024, 11(3): 817-828.
[60]Mease P J, Gottlieb A B, Van Der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase Ⅲ study in psoriatic arthritis[J]. Ann Rheum Dis, 2017, 76(9): 1550-1558.
[61]Mease P, Genovese M C, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase Ⅱ trial[J]. Arthritis Rheum, 2011, 63(4): 939-948.
[62]Østergaard M, Bird P, Pachai C, et al. Implementation of the OMERACT psoriatic arthritis magnetic resonance imaging scoring system in a randomized phase Ⅱb study of abatacept in psoriatic arthritis[J]. Rheumatology (Oxford), 2022, 61(11): 4305-4313.
[63]Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis[J]. N Engl J Med, 2017, 377(16): 1537-1550.
[64]McInnes I B, Anderson J K, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis[J]. N Engl J Med, 2021, 384(13): 1227-1239.
[65]Mease P J, Lertratanakul A, Anderson J K, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2[J]. Ann Rheum Dis, 2021, 80(3): 312-320.
[66]Fleischmann R, Pangan A L, Song I H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase Ⅲ, double-blind, randomized controlled trial[J]. Arthritis Rheumatol, 2019, 71(11): 1788-1800.
[67]Mease P, Coates L C, Helliwell P S, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial[J]. Lancet, 2018, 392(10162): 2367-2377.
[68]Reinisch W, Hellstrom W, Dolhain R J E M, et al. Effects of filgotinib on semen parameters and sex hormones in male patients with inflammatory diseases: results from the phase 2, randomised, double-blind, placebo-controlled MANTA and MANTA-RAy studies[J]. Ann Rheum Dis, 2023, 82(8): 1049-1058.
[69]Pelechas E, Kaltsonoudis E, Migkos M P, et al. State of the art review on the treatment of psoriatic disease[J]. Mediterr J Rheumatol, 2024, 35(1): 66-72.
[70]Ytterberg S R, Bhatt D L, Mikuls T R, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis[J]. N Engl J Med, 2022, 386(4): 316-326.
[71]Charles-Schoeman C, Buch M H, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance[J]. Ann Rheum Dis, 2023, 82(1): 119-129.
[72]Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase Ⅲ and long-term extension studies of tofacitinib in patients with rheumatoid arthritis[J]. Arthritis Rheumatol, 2019, 71(9): 1450-1459.
[73]Szekanecz Z, Giles J T, Buch M H, et al. POS0110 Incidence of major adverse cardiovascular events stratified by geographic region and baseline cardiovascular risk: a post hoc analysis of oral surveillance[J]. Ann Rheum Dis, 2022, 81(S1): 278-279.
[74]Szekanecz Z, Buch M H, Charles-Schoeman C, et al. Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician[J]. Nat Rev Rheumatol, 2024, 20(2): 101-115.
[75]Charles-Schoeman C, Fleischmann R, Mysler E, et al. Risk of venous thromboembolism with tofacitinib versus tumor necrosis factor inhibitors in cardiovascular risk-enriched rheumatoid arthritis patients[J]. Arthritis Rheumatol, 2024, 76(8): 1218-1229.
[76]Mease P J, Deodhar A A, Van Der Heijde D, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase Ⅱ trial in psoriatic arthritis[J]. Ann Rheum Dis, 2022, 81(6): 815-822.
[77]Mease P, Helliwell P, Silwinska-Stanczyk P, et al. Efficacy and safety of the TYK2/JAK1 inhibitor brepocitinib for active psoriatic arthritis: a phase Ⅱb randomized controlled trial[J]. Arthritis Rheumatol, 2023, 75(8): 1370-1380.
猜你喜欢
- 每月28日关爱“女性雄激素增多症” 中国红基会将邀专家讲诊断
- European Radiology:双源PCD-CT在冠状动脉支架成像中的应用
- 正确使用眼药水才能做到缓解眼睛疲劳
- 河北大学张金超教授团队《Biomaterials》:线粒体靶向纳米酶在急性肝损伤防治中的研究
- 蹦迪戴护膝、酒配枸杞?朋克养生要不得
- JAMA Network Open:年轻人群结直肠癌的常见临床表现、风险因素和诊断延迟时间
- 运城市口腔卫生学校附属口腔医院脉动真空灭菌器等设备采购公告
- 味道鲜美的酱炒鸡的做法-香辣味炒菜谱
- 奥密克戎蔓延欧美圣诞节“难过”
- Eur J Med Chem:航天中心医院王昱团队最新综述阐明靶向铁死亡治疗脓毒症肺损伤
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)