首页 > 医疗资讯/ 正文
[摘要] 细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)4/6抑制剂联合内分泌治疗是激素受体阳性、人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)阴性晚期乳腺癌患者的标准一线治疗方案。CDK4/6抑制剂的出现使患者预后得到进一步提升,但也带来了新的临床问题,如许多患者在治疗后仍会出现进展和耐药,而目前对于CDK4/6抑制剂联合内分泌治疗进展的患者缺乏标准的后续治疗方案。内分泌治疗药物耐药通过雌激素受体(estrogen receptor,ESR)依赖或非依赖性途径导致肿瘤进展,新型内分泌治疗药物可能使携带ESR1基因突变的乳腺癌患者获益。携带磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路变异的患者可能对该通路的靶向抑制剂治疗敏感。此外,已获批或正在早期开发的新型抗体药物偶联物(antibody-drug conjugate,ADC)、免疫联合治疗方案及新型细胞周期特异性药物也展现出积极的抗肿瘤活性。以精准药物为基础的组合方案在丰富临床选择的同时,也让个体化治疗成为可能,基于生物标志物的精准治疗策略成为CDK4/6抑制剂后时代的重要发展方向。
[关键词] 激素受体阳性晚期乳腺癌;细胞周期蛋白依赖性激酶4和6抑制剂;精准诊疗;内分泌治疗;靶向治疗;抗体药物偶联物
[Abstract] Cyclin-dependent kinase (CDK)4/6 inhibitors plus endocrine therapy represents the standard first-line treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer. The introduction of CDK4/6 inhibitors has significantly improved the prognosis of breast cancer patients. However, it has also brought new clinical challenges, such as disease progression and treatment resistance in many patients. Currently, there is a lack of standardized subsequent treatment options for patients whose disease progresses after CDK4/6 inhibitor combined with endocrine therapy. Endocrine therapy resistance can lead to tumor progression through estrogen receptor (ESR)-dependent or ESR-independent pathways. Novel endocrine agents have the potential to benefit breast cancer patients harboring ESR1 mutations. Patients with alterations in the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway may be particularly sensitive to targeted inhibitors of this pathway. Furthermore, newly approved or investigational antibody-drug conjugate (ADC), immunotherapy-based combinations, and novel cell cycle inhibitors have demonstrated promising anti-tumor activities. Precision medicine-based combination strategies not only expand clinical treatment options but also enable physicians to make personalized treatment decisions for patients. Biomarker-driven precision therapeutic strategies have emerged as a critical area of treatment development in the post-CDK4/6 inhibitor era.
[Key words] Hormone receptor-positive advanced breast cancer; Cyclin-dependent kinase 4 and 6 inhibitor; Precision medicine; Endocrine therapy; Targeted therapy; Antibody-drug conjugate
乳腺癌是全球女性中发病率最高的恶性肿瘤,其中激素受体阳性、人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)阴性乳腺癌是最常见的亚型,占全部乳腺癌的60%~70%[1]。目前,细胞周期蛋白依赖性激酶(cyclin-dependent kinase,CDK)4/6抑制剂联合内分泌治疗是激素受体阳性晚期乳腺癌的标准一线治疗方案。CDK4/6抑制剂通过阻断细胞周期从G1期到S期的进程,有效地抑制乳腺癌细胞增殖[2]。多项临床研究[3-5]证实,CDK4/6抑制剂联合内分泌治疗相比内分泌单药治疗可显著延长激素受体阳性/HER2-晚期乳腺癌患者的无进展生存期(progression-free survival,PFS)。接受CDK4/6抑制剂治疗的患者仍然会出现耐药,导致疾病进展。对于CDK4/6抑制剂治疗耐药的机制,目前已有多项研究[6-8]证实,细胞周期相关通路变异是导致耐药性的主要机制之一,包括视网膜母细胞瘤易感基因1(retinoblastoma gene 1,RB1)缺失、细胞周期蛋白E1基因(cyclin E1,CCNE1)/CCNE2扩增及CDK6高表达等。磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路变异是CDK4/6抑制剂耐药的另一个重要机制,约50%的激素受体阳性/HER2-乳腺癌患者存在PI3K/AKT/PTEN通路相关基因改变或活化[9]。在全球人群中,PIK3CA突变率为31%~40%,AKT1基因突变率约为5%,PTEN基因变异率约为5%[10-12]。有研究[13-15]显示,这3个基因的改变是乳腺癌患者的独立不良预后因子,且该通路变异是激素受体阳性晚期乳腺癌患者对多种疗法(如内分泌治疗和化疗等)耐药的主要因素之一。因此,精准靶向PI3K/AKT/mTOR通路成为CDK4/6抑制剂后时代乳腺癌治疗的关键方向。
目前,已有多项研究证实了PI3K/AKT/mTOR通路抑制剂治疗激素受体阳性晚期乳腺癌的效果,为激素受体阳性/HER2-晚期乳腺癌的精准治疗开辟了新的方向。此外,CDK4/6抑制剂的跨线治疗,口服选择性雌激素受体下调剂(selective estrogen receptor downregulator,SERD)药物及抗体药物偶联物(antibody-drug conjugate,ADC)药物等均在CDK4/6抑制剂耐药后患者的治疗中取得积极进展。对于CDK4/6抑制剂耐药后的患者,目前临床上仍缺乏标准治疗方案,其未被满足的治疗需求理应得到充分重视。本文将回顾性分析这些药物的研究数据,以期为CDK4/6抑制剂后时代的乳腺癌精准诊疗提供参考。
1 国内可及的内分泌治疗联合靶向治疗方案
1.1 CDK4/6抑制剂跨线治疗
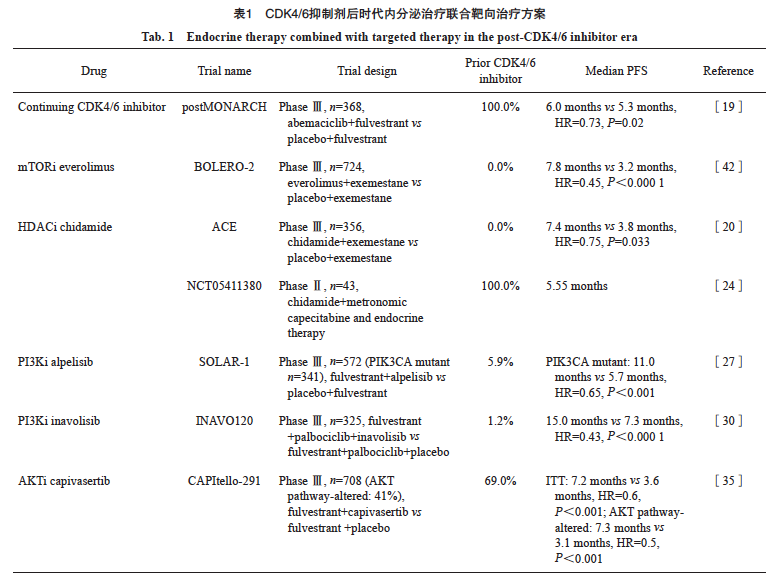
CDK4/6抑制剂用于激素受体阳性/HER2-晚期乳腺癌的一线治疗可显著提高患者的PFS,但对于治疗进展后的患者继续使用CDK4/6抑制剂是否能够获益仍不清楚。目前,多项研究应用CDK4/6抑制剂跨线治疗未取得一致性结论。Ⅱ期PACE研究(中位PFS:4.6个月 vs 4.8个月,HR=1.11,P=0.62)[16]及PALMIRA研究(中位PFS:4.2个月 vs 3.6个月,HR=0.8,P=0.21)[17]设计相似,结果均为阴性。Ⅱ期MAINTAIN研究[18]则取得了阳性结果,瑞波西利组对比内分泌单药组显著改善了PFS(5.3个月 vs 2.8个月,HR=0.57,P=0.006)。Ⅲ期研究postMONARCH[19]进一步验证了CDK4/6抑制剂跨线治疗的获益,该研究纳入晚期一线治疗及早期辅助治疗中接受过CDK4/6抑制剂联合内分泌治疗的患者,超过70%的患者前线接受CDK4/6抑制剂治疗的时间≥12个月,结果显示,阿贝西利联合氟维司群相比氟维司群单药显著改善了PFS (6个月 vs 5.3个月,HR=0.73,P=0.02)。虽然主要终点PFS为阳性结果,但postMONARCH研究中CDK4/6抑制剂跨线治疗的绝对获益仅0.7个月。亚组分析显示,既往CDK4/6抑制剂治疗时间≥12个月的患者(HR=0.70)与<12个月的患者(HR=0.80)获益保持一致,但是<12个月人群的获益趋势更弱。此外,CDK4/6抑制剂跨线治疗对携带PIK3CA/AKT1/PTEN基因变异的亚组HR值为0.86,与通路正常的患者(HR=0.73)相比,获益趋势也较弱[19]。上述结果提示未来需要进一步研究以精确筛选适合CDK4/6抑制剂跨线治疗的患者人群。
1.2 组蛋白去乙酰化酶(histone deacetylase,HDAC)抑制剂
西达苯胺是第一个获批激素受体阳性/HER2-晚期乳腺癌适应证的HDAC抑制剂,其Ⅲ期ACE研究[20]纳入至少接受过1次内分泌治疗后进展的激素受体阳性/HER2-绝经后晚期乳腺癌患者,西达苯胺组的中位PFS为7.4个月,安慰剂组为3.8个月(HR=0.75,P=0.033)。但由于研究进行时,CDK4/6抑制剂尚未普及,该研究未纳入CDK4/6抑制剂治疗失败的患者。在真实世界研究[21-23]中HDAC抑制剂的疗效似乎有限,对CDK4/6抑制剂治疗失败的患者,西达苯胺治疗组的中位PFS仅2.0~4.5个月。
近期,一项Ⅱ期单臂研究[24]评估了在西达苯胺联合内分泌治疗的基础上加入卡培他滨节拍化疗在CDK4/6抑制剂治疗失败的晚期乳腺癌患者中的疗效与安全性,在可评估患者中,中位PFS为5.55个月(95% CI:3.02~10.32)。HDAC抑制剂等三药联合治疗方案仍需要在更大样本量的研究中验证。
1.3 mTOR抑制剂
依维莫司(everolimus)是一种mTOR抑制剂,在Ⅲ期BOLERO-2研究中,与依西美坦联合应用作为二线治疗显著改善了激素受体阳性/ HER2-晚期乳腺癌患者的中位PFS(7.8个月 vs 3.2个月,HR=0.45,P<0.001)[25]。基于该研究结果,everolimus已被批准联合依西美坦用于治疗既往内分泌治疗失败的晚期乳腺癌患者。然而,BOLERO-2研究[25]的总生存期(overall survival,OS)在治疗组与对照组之间差异无统计学意义(31.0个月 vs 26.6个月,HR=0.89,P=0.14)。该研究未纳入经CDK4/6抑制剂治疗的患者,治疗线数相对靠后且无特异性生物标志物指导应用。另一项真实世界研究[26]则提示,依维莫司联合依西美坦在既往使用过CDK4/6抑制剂的患者中的获益显著劣于未曾使用过的患者(中位PFS:3.8个月 vs 5.4个月,HR=1.46,P=0.013)。
2 针对PI3K/AKT/PTEN通路变异患者的精准诊疗方案
2.1 PI3K抑制剂
阿培利司(alpelisib)是一种口服的PI3Kα选择性抑制剂,Ⅲ期SOLAR-1研究[27]探索了alpelisib联合氟维司群对比氟维司群单药在内分泌治疗后进展的激素受体阳性/HER2-晚期乳腺癌患者中的疗效,在携带PIK3CA突变的患者中,实验组的PFS显著延长(中位PFS:11.0个月 vs 5.7个月,HR=0.65,P<0.001),但经CDK4/6抑制剂治疗人群较少(仅5.3%)。Ⅱ期单臂BYLieve研究[28]中,CDK4/6抑制剂联合芳香化酶抑制剂(aromatase inhibitor,AI)治疗后进展且携带PIK3CA突变的患者,使用alpelisib联合氟维司群治疗后的中位PFS为8个月。由于样本量较小,仍需要更大样本的Ⅲ期临床研究来证实。
临床前研究[29]数据显示,同时抑制PI3K、CDK4/6和雌激素受体(estrogen receptor,ESR)3条通路,可能产生协同效应。伊那利塞(inavolisib)是一种新型选择性PI3Kα抑制剂,Ⅲ期INAVO120研究[30]探索了inavolisib+氟维司群+哌柏西利对比氟维司群+哌柏西利的疗效,入组在辅助内分泌治疗期间或治疗结束后1年内快速进展或复发的患者,中期分析表明,三药联合方案较双药方案显著延长了PFS(中位PFS:15.0个月 vs 7.3个月,HR=0.43,P<0.000 1), OS数据待公布,三药联合方案的不良反应值得关注,≥3级的常见不良反应包括中性粒细胞减少症(80.2%)、高血糖(5.6%)、口腔炎或黏膜炎(5.6%)、腹泻(3.7%)等。INAVO120研究主要针对辅助内分泌治疗耐药的人群,经CDK4/6抑制剂治疗患者的比例仅为1.2%,仍缺乏CDK4/6抑制剂治疗失败患者的数据。随着CDK4/6抑制剂在早期乳腺癌辅助强化治疗中的相继获批和应用增加,INAVO120研究适用的获益患者群体将受到限制。此外,其他值得探索的方向包括用药顺序问题(若一线用CDK4/6抑制剂,二线序贯inavolisib,是否能达到同样长的中位PFS2)、二线中探索同时抑制3条通路的三药联合方案的疗效等。
2.2 AKT抑制剂
在激素受体阳性晚期乳腺癌患者中,约一半CDK4/6抑制剂治疗进展患者存在AKT通路变异,AKT是PI3K/AKT/PTEN通路的中心关键节点[9,31]。AKT抑制剂卡帕塞替尼(capivasertib)可抑制3种同工型AKT1/2/3及下游mTOR蛋白,从而阻断整条信号通路[32]。而 PI3K抑制剂可能因受体酪氨酸蛋白激酶(receptor tyrosine kinase,RTK)重新激活,对AKT1突变的细胞不敏感;目前获批的mTOR抑制剂仅抑制mTORC1,未被抑制的mTORC2仍可通过激活AKT导致通路活化[33]。
Ⅱ期FAKTION研究 [34]数据表明,capivasertib联合氟维司群与氟维司群单药相比可显著改善PFS(中位PFS:10.3个月 vs 4.8个月,HR=0.58,P=0.004 4)。随后开展的Ⅲ期CAPItello-291研究[35]纳入了69%接受过CDK4/6抑制剂治疗的患者,结果显示,capivasertib联合氟维司群显著改善了意向治疗(intention-to-treat,ITT)人群的PFS(中位PFS:7.2个月 vs 3.6个月,HR=0.60,P <0.001);PIK3CA/AKT1/PTEN基因变异亚组的PFS也显著获益(中位PFS:7.3个月 vs 3.1个月,HR=0.50,P < 0.001),探索性分析结果表明,在PIK3CA、AKT1、PTEN的任意变异亚组中,PFS获益(HR=0.51、0.51、0.43)呈现一致性,提示capivasertib联合氟维司群的治疗策略可覆盖携带任意一种PIK3CA、AKT1、PTEN基因变异的患者群体,获得显著的预后改善。此外,对于CDK4/6抑制剂耐药的患者,capivasertib疗效与既往CDK4/6抑制剂治疗时长无关,无论治疗时长是否超过12个月,capivasertib治疗组一致获益。接受capivasertib联合氟维司群治疗的患者整体耐受性良好,特别关注的不良反应如≥3级高血糖和口腔炎的发生率分别为2.3%和2.0%,因不良反应导致的停药率为13%[35]。基于此研究结果,美国食品药品管理局(Food and Drug Administration,FDA)批准了capivasertib联合氟维司群治疗存在一种或多种PIK3CA/AKT1/PTEN基因变异的晚期乳腺癌患者。内分泌治疗联合靶向治疗用于CDK4/6抑制剂治疗后疾病进展的乳腺癌患者的数据汇总见表1。
在基因检测策略上,二代测序(next-generation sequencing,NGS)技术以其高通量、可一次性检出各类型变异包括已知及未知变异、单碱基成本低等优点而被广泛认可,各相关检测panel均全面覆盖了PIK3CA、AKT1激活突变以及PTEN失活变异[36]。CAPItello-291研究[35]应用NGS检测,覆盖PIK3CA/AKT1/PTEN基因的33个突变位点,以及导致PTEN蛋白功能缺失的重排和拷贝数纯合缺失等变异,并带来显著的临床获益。若NGS不可及时,聚合酶链反应(polymerase chain reaction,PCR)技术同样成熟,可能作为NGS的一种有效补充手段。另外,美国国立综合癌症网络(National Comprehensive Cancer Network,NCCN)指南[37]、美国临床肿瘤学会(American Society of Clinical Oncology,ASCO)指南[38]和欧洲肿瘤内科学会(European Society for Medical Oncology,ESMO)指南[39]均建议激素受体阳性乳腺癌患者在诊断转移性疾病时活检并进行PIK3CA/AKT/PTEN通路基因分析,活检可提供新的组织样本以确认诊断和进行生物标志物检测。当新鲜组织样本不可及时应优先考虑使用存档的手术或活检组织;如果无法获得组织样本,液体活检[循环肿瘤DNA (circulating tumor DNA,ctDNA)等]也是一种可行的替代选择,在液体活检结果阴性时需补充组织检测[40-41]。
3 口服SERD药物
在晚期乳腺癌患者中ESR1突变频率达30%~40%[43],ESR1属于获得性突变,是CDK4/6抑制剂联合AI耐药的机制之一[44]。传统SERD药物氟维司群可克服ESR1突变[45],但受限于其剂型和药代动力学因素,往往给患者的临床使用带来某些不便。新型口服SERD药物,又称ESR1突变抑制剂,生物利用度更高,有望进一步改善ESR1突变患者的临床获益[46]。对CDK4/6抑制剂治疗后进展合并ESR1突变的患者,换用口服SERD药物是合理的治疗方案。
3.1 艾拉司群(elacestrant)
Elacestrant是第一个获得美国FDA批准的口服SERD药物,Ⅲ期EMERALD研究[47]比较了elacestrant单药与标准内分泌治疗在晚期ESR+乳腺癌中的疗效,患者均为既往接受CDK4/6抑制剂联合AI或氟维司群治疗后疾病进展的患者,结果显示,elacestrant显著改善了患者的中位PFS (中位PFS:2.79个月 vs 1.91个月,HR=0.697,P=0.001 8)。对于ESR1基因突变患者亦观察到显著的PFS改善(中位PFS:3.78个月 vs 1.87个月,HR=0.546,P=0.000 5)。基于此结果,美国FDA批准了elacestrant用于治疗既往至少接受过1种内分泌治疗后疾病进展的ESR1突变ESR+/HER2-晚期或转移性乳腺癌患者。
3.2 Imlunestrant
Imlunestrant是一种新型口服SERD药物,Ⅲ 期EMBER-3研究[48]评估了imlunestrant单药±阿贝西利或标准内分泌单药(依西美坦/氟维司群)治疗接受过内分泌治疗的晚期乳腺癌患者的疗效和安全性,其中59.8%的患者既往接受过CDK4/6抑制剂治疗,与标准内分泌治疗方案相比,imlunestrant单药显著改善了ESR1突变人群的PFS(中位PFS:5.5个月 vs 3.8个月,HR=0.63,P <0.001),在ITT人群中则未取得显著预后改善的效果(中位PFS:5.6个月 vs 5.5个月, HR=0.87,P =0.012)。此外,imlunestrant联合阿贝西利相比imlunestrant单药治疗显著改善了ITT人群的PFS(中位PFS:9.4个月 vs 5.5个月,HR=0.57,P <0.001),亚组分析提示无论是否伴有ESR1突变的患者,获益趋势与ITT人群保持一致。
3.3 Camizestrant
Camizestrant是另一种口服SERD药物,在临床前模型中表现出良好的抗肿瘤活性[49]。SERENA-2研究[50]评估了camizestrant单药对比氟维司群的疗效,该研究入组的患者约50%既往接受过CDK4/6抑制剂治疗,在ITT人群中,与氟维司群相比,75 mg camizestrant(中位PFS:7.2个月 vs 3.7个月,HR=0.58,P=0.012)和150 mg camizestrant(中位PFS:7.7个月 vs 3.7个月, HR=0.67,P=0.016)均可显著改善PFS。在各剂量亚组及ESR1突变或野生型亚组中camizestrant均取得一致的PFS获益。探索性分析显示,camizestrant在抑制ESR1方面优于氟维司群,在ESR1突变ctDNA水平降低≥50%的患者中,camizestrant的中位PFS达11.1个月。两种剂量camizestrant均耐受良好,常见不良反应包括轻度的心动过缓(13.6%)和视觉障碍(18.4%)等。
这些研究进一步明确了这些口服SERD在ESR1突变、激素受体阳性乳腺癌中的作用。将来的研究应针对ESR1突变、激素受体阳性乳腺癌,探索口服SERD与其他靶点抑制剂的联合,如PAM通路抑制剂和CDK4/6抑制剂的跨线 治疗等。
4 ADC在内分泌治疗耐药或不能从内分泌治疗获益患者中的应用
4.1 靶向HER2的ADC
德曲妥珠单抗(trastuzumab deruxtecan, T-DXd)是一种靶向HER2的ADC。DESTINY-Breast04研究[51]比较了T-DXd与医师选择的化疗在不可切除或转移性HER2低表达乳腺癌患者中的疗效,入组包含70%既往使用过CDK4/6抑制剂治疗的患者,结果显示,激素受体阳性人群中,T-DXd可显著改善PFS(中位PFS:10.1个月 vs 5.4个月,HR=0.51,P<0.001)和OS(中位OS:23.9个月 vs 17.5个月,HR=0.64,P=0.003)。T-DXd相比化疗组发生的≥3级治疗相关不良反应更少,发生率分别为52.6%和67.4%。基于DESTINY-Breast04研究突出的疗效与安全性,T-DXd获批用于既往接受过化疗的晚期HER2低表达乳腺癌患者。DESTINY-Breast06研究[52]探索了T-DXd在内分泌治疗耐药的晚期HER2低表达或HER2超低表达乳腺癌患者中的应用,研究纳入了既往接受≥二线内分泌治疗联合靶向治疗或CDK4/6抑制剂联合内分泌治疗≤6个月出现进展的转移性乳腺癌患者,结果显示,T-DXd显著延长了HER2低表达患者的PFS (中位PFS:13.2个月 vs 8.1个月,HR=0.62,P<0.000 1),HER2超低表达亚组患者的PFS也有改善趋势(中位PFS:13.2个月 vs 8.3个月,HR=0.78),提示T-DXd可作为接受至少二线内分泌治疗或一线CDK4/6抑制剂治疗快速进展患者的治疗选择。在激素受体阳性乳腺癌方面,包括RC48-ADC、MRG002、SHR-A1811及A166在内的新型抗HER2 ADC正处于研发阶段,有望在未来为临床治疗提供更多样化的选择[53-56]。
4.2 靶向滋养细胞表面抗原2(trophoblast cell surface antigen 2,TROP2)的ADC
人TROP2与肿瘤细胞增殖及侵袭相关,在激素受体阳性/HER2-乳腺癌中有广泛的表达[57]。戈沙妥珠单抗(sacituzumab govitecan,SG)是一种靶向TROP2的ADC。Ⅲ期TROPiCS-02研究[58]评估了SG在内分泌治疗耐药、激素受体阳性/HER2-晚期乳腺癌患者中的疗效,患者既往接受过至少1种内分泌治疗与CDK4/6抑制剂治疗以及二到四线晚期化疗。与医师选择的化疗相比,SG显著延长了PFS(中位PFS:5.5个月 vs 4.0个月,HR=0.66,P=0.000 3)和OS(14.4个月 vs 11.2个月,HR=0.7,P=0.02)。
Datopotamab deruxtecan(Dato-DXd)是另一种靶向TROP2的ADC,Ⅰ期TROPION-PanTumor01研究[59]评估了Dato-DXd在既往接受过多线治疗的ESR+/HER2-转移性乳腺癌患者中的疗效,其中95%的患者既往接受过CDK4/6抑制剂治疗,结果显示,Dato-DXd的临床获益率(clinical benefit rate,CBR)为41%。Ⅲ期TROPION-Breast01研究[60]进一步比较了Dato-DXd与医师选择的化疗在内分泌治疗失败且接受过一、二线化疗的激素受体阳性/HER2-转移性乳腺癌患者中的疗效,结果显示,Dato-DXd与化疗相比显著延长了患者的PFS(中位PFS:6.9个月 vs 4.9个月,HR=0.63,P<0.000 1),3级及以上不良反应的发生率降低(20.8% vs 44.7%),3~4级中性粒细胞减少的发生率下降尤为显著(1% vs 31%)。在中国患者队列中,Dato-DXd的疗效与全球整体人群一致,均能显著延长PFS(8.1个月 vs 4.2个月,HR=0.54,P=0.032 9)[61]。此外,一系列新型TROP2 ADC正在研究中,芦康沙妥珠单抗已获批用于治疗晚期三阴性乳腺癌,在激素受体阳性乳腺癌领域,芦康沙妥珠单抗仍处于研究阶段,同时ESG401、SHR-A1921等TROP2 ADC亦在研发进程中,预期将丰富该领域的治疗选择[62-64]。
4.3 其他靶点的ADC
除了HER2和TROP2,更多新靶点的ADC正在激素受体阳性乳腺癌领域进行探索。Patritumab Deruxtecan(HER3-DXd)是一种抗HER3 ADC,Ⅱ期单臂ICARUS-BREAST01研究[65]显示,HER3-DXd在经CDK4/6抑制剂治疗进展后的患者中显示出具有临床意义的有效性,中位PFS为9.4个月。此外,靶向Nectin-4的enfortumab vedotin及靶向LIV-1的ladiratuzumab vedotin在激素受体阳性乳腺癌中的早期探索正在积极开展中[66-67]。
5 免疫联合治疗方案的探索
在激素受体阳性早期乳腺癌新辅助治疗中,KEYNOTE-756研究[68]和CheckMate 7FL研究[69]均提示,在化疗的基础上联合免疫检查点抑制剂可显著改善激素受体阳性早期乳腺癌患者的预后。临床前研究[70]显示,ADC与程序性死亡蛋白-1(programmed death-1,PD-1)抑制剂可通过DNA损伤诱导细胞毒性T淋巴细胞募集并减少调节性T细胞的活性。为验证SG与免疫治疗联合是否有协同作用,一项Ⅱ期随机临床试验[71]评估了帕博利珠单抗联合SG在激素受体阳性/HER2-晚期乳腺癌患者中的疗效和安全性,入组患者均接受过至少1次内分泌治疗和0~1次晚期化疗,其中76.9%既往接受过CDK4/6抑制剂治疗,结果显示,联合治疗组较SG单药组未改善PFS(中位PFS:8.1个月 vs 6.2个月,P=0.37)和OS(mOS:18.5个月 vs 18个月,P=0.21),提示需继续探索可能从免疫联合治疗中获益的适宜患者人群。
6 新型内分泌治疗药物和细胞周期特异性药物的研发
一系列具有全新机制的药物正在早期研发阶段,包括新型内分泌治疗药物和细胞周期特异性药物,这些药物在临床试验中显示出一定的抗肿瘤活性。
Vepdegestrant(ARV-471)一种靶向ESR的蛋白靶向水解嵌合体(proteolysis targeting chimera,PROTAC)药物,在Ⅱ期VERITAC研究[72]的剂量扩展队列中,纳入35例经过至少一线CDK4/6抑制剂治疗失败的后线患者,ITT及ESR1突变人群的中位PFS分别为3.7和5.7个月。在一项1b期研究[73]中,vepdegestrant联合哌柏西利在CDK4/6抑制剂耐药且中位治疗线数为四线的患者中,中位PFS达11.1个月。多项Ⅲ期VERITAC系列研究正在进行单药、联合CDK4/6抑制剂,以及在激素受体阳性晚期前线治疗中的探索。
此外,靶向ESR的新型内分泌治疗药物如选择性雌激素受体共价拮抗剂(selective estrogen receptor covalent antagonist,SERCA)H3B-6545[74]和完全雌激素受体拮抗剂(complete estrogen receptor antagonist,CERAN)palazestrant[75]已在早期临床探索中初步展现出抗肿瘤活性。KAT6A抑制剂是一种靶向表观遗传学致癌靶点的新型药物,一项Ⅰ期研究[76]在经过CDK4/6抑制剂治疗、既往治疗方案的中位线数为二线的患者中获得中位PFS为10.7个月的积极数据。多种新型选择性CDK2和CDK4抑制剂正在早期开发。其中一项Ⅰ/Ⅱ期研究[77]中,CDK4抑制剂PF-07220060针对既往接受过二线或以上治疗的激素受体阳性/HER2-晚期乳腺癌患者,该方案的CBR达60.6%,客观缓解率(objective response rate,ORR)为32%。目前评估PF-07220060联合氟维司群对比研究者选择的内分泌治疗的Ⅲ期临床研究正在开展中。
7 总结和展望
综上所述,CDK4/6抑制剂治疗后的治疗策略正处于快速发展阶段,尽管当前尚缺乏统一的标准治疗路径,多种潜在方案正在积极探索中。PIK3CA/AKT/PTEN通路基因改变与激素受体阳性/HER2-晚期乳腺癌的内分泌治疗及CDK4/6抑制剂耐药密切相关,已成为临床验证的生物标志物,靶向该通路中的关键分子在精准治疗中具有广阔前景。Capivasertib联合氟维司群显示出良好的疗效和安全性,可能成为激素受体阳性/HER2-乳腺癌患者的标准二线治疗方案。对于内分泌治疗敏感的患者,如PI3K/AKT/PTEN通路正常,CDK4/6抑制剂跨线治疗是目前合理的选择。对于ESR1突变的患者,口服SERD药物已展现出有潜力的疗效和安全性。而对于内分泌治疗耐药或无法从内分泌治疗中获益的患者,ADC药物提供了坚实的循证医学依据,展现出在延长生存和减少化疗使用方面的临床价值。此外,大量新型内分泌药物和细胞周期特异性药物正在早期研发中,预计未来将为激素受体阳性晚期乳腺癌的治疗提供更多选择。
随着精准检测技术的不断发展和可及性的提升,基因检测的重要性日益增加。对于显著影响患者预后的靶点,如PI3K/AKT/PTEN通路和ESR1等,建议在疾病转移阶段尽早进行基因检测,为患者制订个性化治疗方案。随着相关检测技术的普及和精准靶向药物的不断涌现,未来,CDK4/6抑制剂后时代乳腺癌患者的精准诊疗有望取得更大的进步。
第一作者:
李彬,博士。
通信作者:
胡夕春,博士,主任医师、教授,复旦大学附属肿瘤医院肿瘤内科首席专家。
作者贡献声明:
李彬:资料收集,文章撰写;陶中华:资料收集,写作指导;胡夕春:主题指导,文章撰写和修改。
[参考文献]
[1] PEROU C M, SØRLIE T, EISEN M B, et al. Molecular portraits of human breast tumours[J]. Nature, 2000, 406(6797): 747-752.
[2] FINN R S, ALESHIN A, SLAMON D J. Targeting the cyclindependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers[J]. Breast Cancer Res, 2016, 18(1): 17.
[3] Ribociclib as first-line therapy for HR-positive, advanced breast cancer[J]. N Engl J Med, 2018, 379(26): 2582.
[4] FINN R S, MARTIN M, RUGO H S, et al. Palbociclib and letrozole in advanced breast cancer[J]. N Engl J Med, 2016, 375(20): 1925-1936.
[5] GOETZ M P, TOI M, CAMPONE M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer[J]. J Clin Oncol, 2017, 35(32): 3638-3646.
[6] O’LEARY B, CUTTS R J, LIU Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial[J]. Cancer Discov, 2018, 8(11): 1390-1403.
[7] WANDER S A, COHEN O, GONG X Q, et al. The genomic landscape of intrinsic and acquired resistance to cyclindependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer[J]. Cancer Discov, 2020, 10(8): 1174-1193.
[8] O’LEARY B, CUTTS R J, HUANG X, et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer[J]. J Natl Cancer Inst, 2021, 113(3): 309-317.
[9] MILLIS S Z, IKEDA S, REDDY S, et al. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors[J]. JAMA Oncol, 2016, 2(12): 1565-1573.
[10] SMYTH L M, ZHOU Q, NGUYEN B, et al. Characteristics and outcome of AKT1E17K-mutant breast cancer defined through AACR project GENIE, a clinicogenomic registry[J]. Cancer Discov, 2020, 10(4): 526-535.
[11] RINALDI J, SOKOL E S, HARTMAIER R J, et al. The genomic landscape of metastatic breast cancer: insights from 11 000 tumors[J]. PLoS One, 2020, 15(5): e0231999.
[12] STURGILL E G, MISCH A, LACHS R, et al. Next-generation sequencing of patients with breast cancer in community oncology clinics[J]. JCO Precis Oncol, 2021, 5: 1297-1311.
[13] SOBHANI N, ROVIELLO G, CORONA S P, et al. The prognostic value of PI3K mutational status in breast cancer: a meta-analysis[J]. J Cell Biochem, 2018, 119(6): 4287-4292.
[14] RUDOLPH M, ANZENEDER T, SCHULZ A, et al. AKT1 (E17K) mutation profiling in breast cancer: prevalence, concurrent oncogenic alterations, and blood-based detection[J]. BMC Cancer, 2016, 16: 622.
[15] MOSELE F, STEFANOVSKA B, LUSQUE A, et al. Outcome and molecular landscape of patients with PIK3CA mutated metastatic breast cancer[J]. Ann Oncol, 2020, 31(3): 377-386.
[16] MAYER E L, REN Y, WAGLE N, et al. PACE: A randomized phase Ⅱ study of fulvestrant, palbociclib, and avelumab after progression on cyclin-dependent kinase 4/6 inhibitor and aromatase inhibitor for hormone receptor-positive/human epidermal growth factor receptor-negative metastatic breast cancer[J]. J Clin Oncol, 2024, 42(17): 2050-2060.
[17] LLOMBART-CUSSAC A, HARPER-WYNNE C, PERELLO A, et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): PALMIRA trial[J]. J Clin Oncol, 2023, 41(16_suppl): 1001.
[18] KALINSKY K, ACCORDINO M K, CHIUZAN C, et al. Randomized phase Ⅱ trial of endocrine therapy with or without ribociclib after progression on cyclin-dependent kinase 4/6 inhibition in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: MAINTAIN trial[J]. J Clin Oncol, 2023, 41(24): 4004-4013.
[19] KALINSKY K, BIANCHINI G, HAMILTON E P, et al. Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2- advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: primary outcome of the phase 3 postMONARCH trial[J]. J Clin Oncol, 2024, 42(17_suppl): LBA1001.
[20] JIANG Z F, LI W, HU X C, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptorpositive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2019, 20(6): 806-815.
[21] ZHOU J M, WU X X, ZHANG H Q, et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer[J]. Breast, 2022, 66: 255-261.
[22] SUO J J, ZHU K R, ZHUANG C Y, et al. Efficacy and safety of tucidinostat in patients with advanced hormone receptorpositive human epidermal growth factor receptor 2-negative breast cancer: real-world insights[J]. Ann Transl Med, 2023, 11(12): 409.
[23] YUAN Y, ZHANG S H, WANG T, et al. Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2- metastatic breast cancer[J]. Transl Breast Cancer Res, 2023, 4: 10.
[24] ZHENG Q, ZHOU H, WU H L, et al. 368P Tucidinostat and metronomic capecitabine plus endocrine therapy for patients with HR+/HER2- advanced breast cancer after CDK4/6 inhibitors: Preliminary findings of a multi-center, phase Ⅱ study[J]. Ann Oncol, 2024, 35: S372.
[25] BASELGA J, CAMPONE M, PICCART M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer[J]. N Engl J Med, 2012, 366(6): 520-529.
[26] MO H J, RENNA C E, MOORE H C F, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors[J]. Clin Breast Cancer, 2022, 22(2): 143-148.
[27] ANDRÉ F, CIRUELOS E M, JURIC D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1[J]. Ann Oncol, 2021, 32(2): 208-217.
[28] RUGO H S, LEREBOURS F, CIRUELOS E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study[J]. Lancet Oncol, 2024, 25(12): e629-e638.
[29] HANAN E J, BRAUN M G, HEALD R A, et al. Discovery of GDC-0077 (inavolisib), a highly selective inhibitor and degrader of mutant PI3Kα[J]. J Med Chem, 2022, 65(24): 16589-16621.
[30] JHAVERI K, IM S A, SAURA C, et al. Abstract GS03- 13: inavolisib or placebo in combination with palbociclib and fulvestrant in patients with PIK3CA-mutated, hormone receptor-positive, HER2-negative locally advanced or metastatic breast cancer: phase Ⅲ INAVO120 primary analysis[J]. Cancer Res, 2024, 84 (9_Supplement): GS03-13.
[31] MANNING B D, CANTLEY L C. AKT/PKB signaling: navigating downstream[J]. Cell, 2007, 129(7): 1261-1274.
[32] RIBAS R, PANCHOLI S, GUEST S K, et al. AKT antagonist AZD5363 influences estrogen receptor function in endocrineresistant breast cancer and synergizes with fulvestrant (ICI182780) in vivo[J]. Mol Cancer Ther, 2015, 14(9): 2035-2048.
[33] VITALE S R, MARTORANA F, STELLA S, et al. PI3K inhibition in breast cancer: Identifying and overcoming different flavors of resistance[J]. Crit Rev Oncol Hematol, 2021, 162: 103334.
[34] HOWELL S J, CASBARD A, CARUCCI M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptorpositive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial[J]. Lancet Oncol, 2022, 23(7): 851-864.
[35] TURNER N C, OLIVEIRA M, HOWELL S J, et al. Capivasertib in hormone receptor-positive advanced breast cancer[J]. N Engl J Med, 2023, 388(22): 2058-2070.
[36] LEE H, CHO Y A, KIM D G, et al. Next-generation sequencing in breast cancer patients: real-world data for precision medicine[J]. Cancer Res Treat, 2024, 56(1): 149-161.
[37] GRADISHAR W J, MORAN M S, ABRAHAM J, et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2024, 22(5): 331-357.
[38] HENRY N L, SOMERFIELD M R, DAYAO Z, et al. Biomarkers for systemic therapy in metastatic breast cancer: ASCO guideline update[J]. J Clin Oncol, 2022, 40(27): 3205-3221.
[39] GENNARI A, ANDRÉ F, BARRIOS C H, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer[J]. Ann Oncol, 2021, 32(12): 1475-1495.
[40] SCHWARTZBERG L, KIM E S, LIU D, et al. Precision oncology: who, how, what, when, and when not?[J]. Am Soc Clin Oncol Educ Book, 2017, 37: 160-169.
[41] ROSIN J, SVEGRUP E, VALACHIS A, et al. Discordance of PIK3CA mutational status between primary and metastatic breast cancer: a systematic review and meta-analysis[J]. Breast Cancer Res Treat, 2023, 201(2): 161-169.
[42] PICCART M, HORTOBAGYI G N, CAMPONE M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2[J]. Ann Oncol, 2014, 25(12): 2357-2362.
[43] JESELSOHN R, BUCHWALTER G, DE ANGELIS C, et al. ESR1 mutations: a mechanism for acquired endocrine resistance in breast cancer[J]. Nat Rev Clin Oncol, 2015, 12(10): 573-583.
[44] BRETT J O, SPRING L M, BARDIA A, et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptorpositive breast cancer[J]. Breast Cancer Res, 2021, 23(1): 85.
[45] BIDARD F C, HARDY-BESSARD A C, DALENC F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Oncol, 2022, 23(11): 1367-1377.
[46] NEUPANE N, BAWEK S, GURUSINGHE S, et al. Oral SERD, a novel endocrine therapy for estrogen receptor-positive breast cancer[J]. Cancers (Basel), 2024, 16(3): 619.
[47] BARDIA A, BIDARD F C, NEVEN P, et al. Abstract GS3-01: GS3-01 EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting[J]. Cancer Res, 2023, 83(5_Supplement): GS3-1.
[48] JHAVERI K L, NEVEN P, CASALNUOVO M L, et al. Imlunestrant with or without abemaciclib in advanced breast cancer[J]. N Engl J Med, 2024.
[49] SCOTT J S, MOSS T A, BALAZS A, et al. Discovery of AZD9833, a potent and orally bioavailable selective estrogen receptor degrader and antagonist[J]. J Med Chem, 2020, 63(23): 14530-14559.
[50] OLIVEIRA M, POMINCHUCK D, NOWECKI Z, et al. Abstract GS3-02: GS3-02 camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial[J]. Cancer Res, 2023, 83(5_Supplement): GS3-2-GS3-02.
[51] MODI S N, JACOT W, YAMASHITA T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer[J]. N Engl J Med, 2022, 387(1): 9-20.
[52] CURIGLIANO G, HU X C, DENT R A, et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): primary results from DESTINYBreast06 (DB-06)[J]. J Clin Oncol, 2024, 42(17_suppl): LBA1000.
[53] YAN M, LV H M, NIU L M, et al. Efficacy and safety of HER2- ADC SHR-A1811 in HER2-positive breast cancer with brain metastases[J]. J Clin Oncol, 2024, 42(16_suppl): e13006.
[54] ZHANG J, LIU R J, GAO S P, et al. Phase I study of A166, an antibody-drug conjugate in advanced HER2-expressing solid tumours[J]. NPJ Breast Cancer, 2023, 9(1): 28.
[55] WANG J Y, LIU Y J, ZHANG Q Y, et al. RC48-ADC, a HER2- targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies[J]. J Clin Oncol, 2021, 39(15_suppl): 1022.
[56] JIANG Z F, SUN T, WANG X J, et al. A multiple center, open-label, single-arm, phase Ⅱ clinical trial of MRG002, an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer[J]. J Clin Oncol, 2022, 40(16_suppl): 1102.
[57] VIDULA N, YAU C, RUGO H. Trophoblast cell surface antigen 2 gene (TACSTD2) expression in primary breast cancer[J]. Breast Cancer Res Treat, 2022, 194(3): 569-575.
[58] RUGO H S, BARDIA A, MARMÉ F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet, 2023, 402(10411): 1423-1433.
[59] MERIC-BERNSTAM F, KROP I, JURIC D, et al. Abstract PD13-08: PD13-08 phase 1 TROPION-PanTumor01 study evaluating datopotamab deruxtecan (dato-DXd) in unresectable or metastatic hormone receptor-positive/HER2 negative breast cancer (BC)[J]. Cancer Res, 2023, 83(5_Supplement): PD13-8-PD13-08.
[60] BARDIA A, JHAVERI K, IM S A, et al. LBA11 Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2-) breast cancer (BC): primary results from the randomised phase Ⅲ TROPION-Breast01 trial[J]. Ann Oncol, 2023, 34: S1264-S1265.
[61] WANG S, ZHANG Q, JIANG Z, et al. 38MO Datopotamab deruxtecan (Dato-DXd) vs chemotherapy (CT) in patients (pts) with pre-treated inoperable/metastatic hormone receptorpositive, HER2-negative (HR+/HER2-) breast cancer (BC): results from TROPION-Breast01 China cohort[J]. Ann Oncol, 2024, 35: S1418-S1419.
[62] OUYANG Q, YIN Y, SONG L, et al. 380MO SKB264 (MK-2870) in previously treated hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer (mBC): results from a phase Ⅰ/Ⅱ, single-arm, basket trial[J]. Ann Oncol, 2023, 34: S337.
[63] ZHAO J, HUANG F B, XU X, et al. Case report: Prolonged benefit of ESG401, a Trop2 antibody-drug conjugate, in endocrine-refractory hormone receptor-positive, HER-2 negative metastatic breast cancer[J]. Front Oncol, 2024, 14: 1444431.
[64] TONG Y J, FAN X B, LIU H, et al. Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: mechanisms, clinical applications, and future directions[J]. Front Immunol, 2024, 15: 1495675.
[65] PISTILLI B, PIEROTTI L, LACROIX-TRIKI M, et al. 340O Efficacy, safety and biomarker analysis of ICARUS BREAST01: a phase Ⅱ study of patritumab deruxtecan (HER3-DXd) in patients (pts) with HR+/HER2- advanced breast cancer (ABC)[J]. Ann Oncol, 2024, 35: S357.
[66] MEDFORD A J, NIEMIERKO A, ABELMAN R O, et al. Nectin-4 expression in primary breast cancer and associated clinical outcomes[J]. J Clin Oncol, 2024, 42(16_suppl): 570.
[67] CAO A T, HIGGINS S, STEVENS N, et al. Abstract 2742: additional mechanisms of action of ladiratuzumab vedotin contribute to increased immune cell activation within the tumor[J]. Cancer Res, 2018, 78(13_Supplement): 2742.
[68] CARDOSO F, JIA L, HIRSHFIELD K, et al. KEYNOTE-756: Randomized, double-blind, phase Ⅲ study of pembrolizumab vs placebo+neoadjuvant chemotherapy (CT) and adjuvant endocrine therapy (ET) for high-risk, early-stage estrogen receptor–positive human epidermal growth factor receptor 2-negative (ER+/HER2–) breast cancer (BC)[J]. Ann Oncol, 2019, 30: iii38.
[69] LOI S, MCARTHUR H L, HARBECK N, et al. A phase Ⅲ trial of nivolumab with neoadjuvant chemotherapy and adjuvant endocrine therapy in ER+/HER2- primary breast cancer: CheckMate 7FL[J]. J Clin Oncol, 2020, 38(15_suppl): TPS604.
[70] IWAI T, SUGIMOTO M, WAKITA D, et al. Topoisomerase Ⅰ inhibitor, irinotecan, depletes regulatory T cells and upregulates MHC class Ⅰ and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti- PD-L1 antibodies[J]. Oncotarget, 2018, 9(59): 31411-31421.
[71] GARRIDO-CASTRO A C, KIM S E, DESROSIERS J, et al. SACI-IO HR+: a randomized phase Ⅱ trial of sacituzumab govitecan with or without pembrolizumab in patients with metastatic hormone receptor-positive/HER2-negative breast cancer[C]. Chicago: ASCO, 2024.
[72] SCHOTT A F, HURVITZ S, MA C, et al. Abstract GS3- 03: GS3-03 ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: phase 2 expansion (VERITAC) of a phase 1/2 study[J]. Cancer Res, 2023, 83(5_Supplement): GS3-3-GS3-03.
[73] HAMILTON E, JESELSOHN R, HURVITZ S, et al. Abstract PS15-03: vepdegestrant, a PROteolysis TArgeting chimera (PROTAC) estrogen receptor (ER) degrader, plus palbociclib in ER-positive/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer: phase 1b cohort[J]. Cancer Res, 2024, 84(9_Supplement): PS15-3-PS15-03.
[74] HAMILTON E P, WANG J S, PLUARD T J, et al. Phase Ⅰ/Ⅱ study of H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer[J]. J Clin Oncol, 2021, 39(15_suppl): 1018.
[75] PARISIAN A D, BARRATT S A, HODGES-GALLAGHER L, et al. Palazestrant (OP-1250), a complete estrogen receptor antagonist, inhibits wild-type and mutant ER-positive breast cancer models as monotherapy and in combination[J]. Mol Cancer Ther, 2024, 23(3): 285-300.
[76] MUKOHARA T, PARK Y H, SOMMERHALDER D, et al. A phase 1 dose expansion study of a first-in-class KAT6 inhibitor (PF-07248144) in patients with advanced or metastatic ER+ HER2-breast cancer[J]. J Clin Oncol, 2024, 42(16_suppl): 3006.
[77] YAP T A, GIORDANO A, HAMILTON E P, et al. First-inhuman first-in-class phase 1/2a study of the next generation CDK4-selective inhibitor PF-07220060 in patients (pts) with advanced solid tumors, enriched for HR+ HER2- mBC who progressed on prior CDK4/6 inhibitors and endocrine therapy[J]. J Clin Oncol, 2023, 41(16_suppl): 3009.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)