首页 > 医疗资讯/ 正文
综 述
抑郁障碍(MDD)是一种常见的精神障碍,以持续的情绪低落和/或快感缺失为主要表现,常伴有睡眠障碍、疲劳、注意力下降等症状[1-2]。多数患者表现为发作性病程,并对现有抗抑郁药物治疗敏感,但仍有高达1/3的患者对至少2种不同的抗抑郁药治疗响应不佳,症状持续或恶化,发展为难治性抑郁(TRD)[3]。此外,MDD复发风险高,首次发作后的复发率可达50%~60%,而3次及以上发作的患者复发率则高达90%[4]。TRD和MDD高复发率不仅严重影响患者的生活质量,也显著增加了医疗成本[5]。
近年来,重复经颅磁刺激(rTMS)治疗因其非侵入性、有效性和安全性受到广泛关注。2008年,美国食品药品监督管理局(FDA)批准rTMS用于治疗TRD[6],加拿大情绪和焦虑治疗网络将rTMS推荐为TRD的一线治疗方法[7]。科学研究与临床实践中,rTMS通常包括急性治疗和维持性治疗两种疗程。已有研究证实rTMS急性治疗具有显著的抗抑郁效果[8-10],但其疗效的影响因素和长期可持续性仍不明确。此外,rTMS维持治疗的治疗方案和其复发预防作用亦尚无定论。本文旨在回顾rTMS急性和维持性治疗在MDD中的最新应用研究进展,探讨rTMS的抗抑郁作用及未来应用潜力。
1 rTMS急性治疗
rTMS用于MDD急性期治疗的原理在于通过磁场诱发局部电活动,调节大脑皮层功能,从而快速缓解抑郁症状。根据MDD前额叶不对称理论,患者常表现为左侧背外侧前额叶皮质(DLPFC)活动减少,右侧DLPFC活动过度[11]。因此,急性期rTMS治疗通常采用高频(≥10 Hz,HF-rTMS)刺激左侧DLPFC以增强其兴奋性,使用低频(≤1 Hz,LF-rTMS)刺激右侧DLPFC以抑制其过度活动[12]。这种调节机制被认为是rTMS治疗MDD的核心原理。
1.1 疗效和影响因素
研究已证实,rTMS急性治疗可缓解抑郁相关症状[13],降低自杀意念[14],并改善患者的生活质量和功能状态[15]。鉴于其良好的安全性和耐受性,rTMS常作为药物治疗的增效手段应用于临床实践[16]。然而,其总体疗效仍有限,临床研究报告的急性期应答率(29%~46%)和缓解率(18%~31%)仍有较大提升空间[17-18]。影响rTMS疗效的因素涉及以下多个方面。
1.1.1 刺激方案
美国 FDA批准针对左侧 DLPFC的HF-rTMS治疗方案通常为:每天1次,持续4~6周,1个疗程总治疗次数为20~30次[19]。虽然右侧LF-rTMS的抗抑郁疗效证据尚不充分且未获FDA批准,但有荟萃分析显示右侧LF-rTMS和左侧HF-rTMS的疗效相当[20]。值得注意的是,双侧顺序刺激方案在大型回顾性研究(n>3000)中未显现出疗效优势[21]。现有证据提示,HF-rTMS可能加速抗抑郁治疗反应,而LF-rTMS具有更好的耐受性[22]。有学者认为右侧LF-rTMS治疗对于焦虑较严重的患者更具优势,但目前的证据尚不支持这一观点[23]。
1.1.2 刺激参数
rTMS的参数优化可能是提升疗效的关键。在单次脉冲数方面,较高剂量(5625脉冲)的HF-rTMS相对于标准剂量(2250脉冲)显示出更高的应答率[24],可能较高的脉冲剂量能够提高皮质兴奋性[25]。针对总治疗次数的研究显示,5~20次范围内的治疗次数增加与疗效提升相关[26];治疗次数超过36次的患者可进一步改善抑郁症状[27]。荟萃分析发现,日治疗次数(1~10次)和日脉冲数(250~18 000脉冲)与疗效正相关;但单次脉冲数(250~8000脉冲)、疗程总脉冲数(1250~160 000脉冲)和疗程天数(3~30 d)并不存在剂量-反应效应[28]。包含65项随机对照试验(RCT)的荟萃分析发现,当同时纳入HF/LF-rTMS研究时,单次脉冲数和疗程总次数与疗效之间的关联并不显著[29]。上述不一致的发现提示rTMS参数与疗效之间可能存在非线性剂量-反应关系。最近Hsu等[30]研究表明,刺激频率是影响rTMS疗效的关键因素,与其他参数的交互作用可能会显著影响最终治疗效果。因此提出“高频(>10 Hz)、高强度(>100% 运动阈值)、足量脉冲”可能构成最佳参数组合。
1.1.3 年龄/性别
年龄是最常见的预测因素。年龄越小的患者可能获得更好的rTMS治疗效果[31];在青少年MDD(12~21岁)中的发现支持这一结论[32-33]。另一项研究结果显示,成年患者(18~59岁)相比于老年患者(≥60岁)在接受rTMS治疗后的缓解率更高[33]。然而大型回顾性研究(n=5010)结果不认为rTMS的疗效会随着年龄的增长而下降[34]。这种分歧可能与一些研究的治疗周期较短有关[32-33],当疗程更长时(≥6周),老年患者则与年轻患者疗效相当,但老年人的治疗反应较慢[35]。性别差异的研究结果较为一致,女性患者普遍显示出更好的治疗应答[34-36]。
1.1.4 临床特征
基线抑郁症状严重程度可能预示更好的rTMS治疗效果[37],青少年MDD患者的研究也支持这一发现[32]。但在MDD共病创伤后应激障碍(PTSD)患者中,严重抑郁反而与疗效呈负相关[38]。针对共病PTSD的研究尚未达成一致结论,研究对象为退伍军人(MDD合并PTSD)的RCT结果显示,rTMS(相较于伪刺激)不能显著提高抑郁缓解率[39];但基于自然队列的研究结果支持rTMS对该群体的有效性[40]。合并强迫症[41]和边缘型人格障碍[42]似乎不影响rTMS对MDD的治疗效果。病程特征方面,研究一致认为病程较长通常与较差的治疗结果相关[43],而当前发作持续时间较短与更好的治疗效果相关[44],提示尽早考虑使用rTMS可能获益更大。
1.1.5 合并用药和治疗抵抗性
rTMS与抗抑郁药联合使用可能比单独使用rTMS 效果更佳[45-46],但对于苯二氮类药物(如劳拉西泮),回顾性研究发现其可能会阻碍患者对rTMS的治疗反应[47-48],但这一结论需要在前瞻性、基于假设的治疗研究中进一步验证。早期一项多中心RCT研究认为,较高的药物治疗抵抗性与较差的治疗效果相关[44]。近年来的RCT研究认为,4周标准的rTMS治疗对严重难治性抑郁症患者无效[49]。
总体而言,女性、抗抑郁药物联合使用考虑为rTMS疗效的积极因素;苯二氮类药物和较长的病程考虑为rTMS疗效的消极因素;其余因素(包括年龄、基线抑郁水平等)呈现出混杂和不一致的证据水平[43],提示在寻找rTMS抗抑郁治疗响应的决定性影响因子方面仍存在较大挑战。
1.2 疗效的长期持续性及影响因素
尽管rTMS急性治疗已被证明能够有效缓解抑郁症状,但其抗抑郁效果能否持续以及持续多久,现有研究表现出复杂的时效特征。短期随访(≤16周)数据显示,2周急性期治疗后,即使不增加额外的rTMS干预,也能在随访期间继续观察到抗抑郁效果,甚至出现“晚期效应”:真伪刺激组在治疗结束时的疗效差异较小,但在随访12周时逐渐扩大[50]。
一项研究描述了MDD患者从接受治疗到停止治疗的症状变化对数曲线:抑郁症状在前3~4周快速缓解,之后保持缓慢而稳定的改善直至第16周[51]。这种维持模式可能与神经可塑性延迟效应相关。然而,中长期随访研究却认为急性期rTMS的抗抑郁效果会随时间推移而减弱:66.5%的患者在3个月内治疗效应得以保持,但这一比例在治疗后12个月降至46.3%[52]。Cohen等[53]追踪204例接受rTMS治疗并缓解的患者发现,6个月内的复发率高达80%。也有研究估计,对rTMS急性治疗有反应的患者,其效果持续时间约为5个月,此时复发率上升至 20%[54]。综合现有证据,rTMS的持续疗效可能呈现阶段性衰减特征,即急性期效果可维持3~5个月,但超过此时间窗后,单靠急性期治疗难以有效预防复发。
1.2.1 急性期治疗参数与疗效维持
rTMS急性治疗参数中,较少的治疗次数与较高的复发风险有关,而其他rTMS刺激参数与复发并无显著相关性[55]。此外,rTMS治疗结束后抑郁症状的缓解程度和功能改善与早期复发率之间存在显著相关性。具体而言,治疗后症状缓解和功能改善越显著,早期复发率越低[56];相反,症状改善越不明显的患者复发可能性越大[55]。上述发现强调了应以最大程度缓解抑郁症状和改善功能为目标,在急性期为患者提供足量rTMS治疗。
1.2.2 患者特征与复发风险
人口学因素对复发的影响呈现异质性。在年龄维度上,有短期随访研究表明,年龄较大的患者更易复发[50,53],但随访窗口更长(3个月和6个月)的荟萃分析未见年龄与复发风险存在关联[52],提示年龄可能仅在治疗结束后的短时间内对复发有影响。与急性期疗效存在性别差异的发现不同,性别因素在疗效持续性研究中始终未显示预测价值[55]。共病焦虑可能与复发风险相关。Rosenberg 等[57]发现,与未复发患者相比,复发患者在rTMS治疗前焦虑水平较低,但在治疗后焦虑加重。Richieri等[56]也发现,复发患者在治疗结束时焦虑症状更严重。
2 rTMS维持性治疗
rTMS维持性治疗是指在rTMS急性治疗结束后,为达到临床缓解或抑郁症状较轻的患者提供间歇性、长期的rTMS治疗,以巩固急性期治疗达到的症状和功能缓解水平[19],有研究者将其称为保持性rTMS(preservation rTMS)[58]。
既往研究探索了多种维持治疗方案,包括随时间递减的维持治疗方案,以及密集式rTMS维持治疗方案。前者较为简单,通常是以每周、每2周或每月进行1次 rTMS 治疗来维持疗效。密集式rTMS维持治疗方案由Fitzgerald等[59]提出,基于在短时间内重复应用rTMS可能获得更好、更持久疗效的假设。在该方案中,患者在急性治疗诱导缓解后,每个月连续2 d接受5次rTMS治疗。然而,目前对于rTMS维持治疗是否必要以及最佳维持治疗方案尚未达成共识。
2.1 rTMS维持治疗对抑郁复发的作用
MDD患者接受rTMS急性治疗后症状的变化轨迹显示,rTMS维持治疗有助于MDD患者在急性治疗结束后的5个月内维持情绪的相对稳定,而未进行维持治疗的患者在12个月的随访期内可能出现中/高度抑郁症状[60]。对急性治疗有反应的患者进行逐渐减量的rTMS维持治疗(从每周3次递减至每月1次,为期20周),发现维持治疗组患者的复发率显著降低[56]。Fitzgerald等[59]也报道密集式rTMS维持治疗可大幅延长缓解期。
然而,三项RCT研究[61-63]对此提出了挑战。这些研究针对TRD患者进行11~12个月的观察,结果显示无论期间是否服用抗抑郁药物,维持治疗组与对照组的终点抑郁症状评分均无显著差异。这一结果可能是由于较小的研究样本量[61]和随访期间较高的脱落率(>70%)[62]所致。
为解决这一争议,Matsuda等[64]对这三项研究进行个体患者数据荟萃分析,发现rTMS维持治疗可降低临床稳定的成人TRD患者的复发率,这一结果在之后的一项大型RCT(n=281)中得到验证[65]:与单纯药物维持组相比,密集式rTMS维持组12个月复发率降低20.2%(24.2% 比 44.4%);且rTMS联合药物维持治疗组显示出叠加效应(复发率15.9%);同时,rTMS维持治疗展示出较好的安全性和耐受性。
2.2 rTMS维持治疗的临床建议
目前,国内外部分专家共识给出了rTMS维持治疗的推荐建议:常规采用药物维持治疗以避免复发,但当患者有频繁复发史(1年内复发2次及以上)时,可考虑采用rTMS维持治疗,具体见表1。
表1 rTMS维持治疗抑郁障碍的专家推荐意见
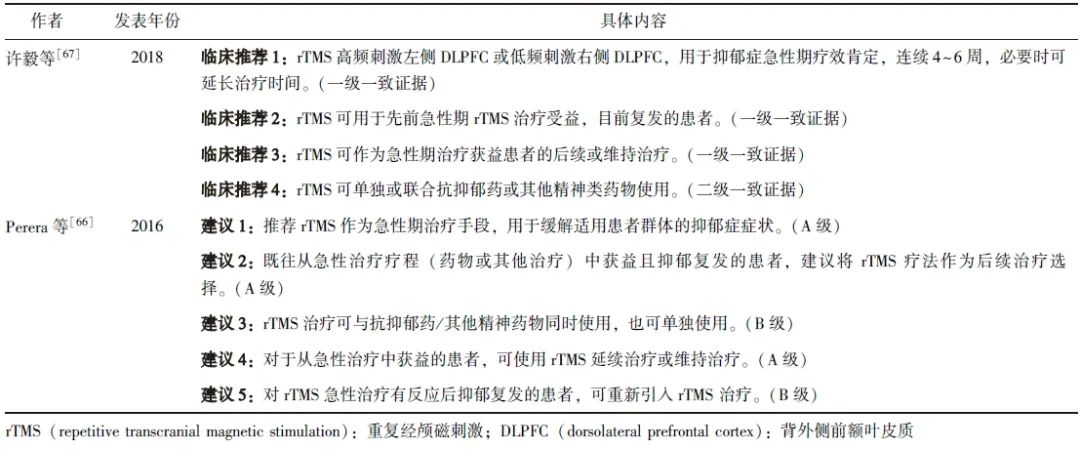
澳大利亚与新西兰精神科医师学会最新发布的关于rTMS的指南也给出了类似建议[19],指出基于目前有限的证据,rTMS维持治疗最明确的适应证是对rTMS急性治疗反应良好且较早出现复发症状的患者。对于该类患者,可在急性治疗后接受第二次急性治疗(rTMS再治疗)以恢复缓解[68],然后进行rTMS维持治疗以延长缓解期。
因此在成功完成rTMS急性疗程后,应定期监测患者抑郁症状。若患者维持缓解期超过1年(期间未接受额外rTMS治疗)后抑郁症状明显复发,则建议行rTMS再治疗,随后进行常规随访。若患者在rTMS急性治疗后1年内出现严重的复发症状,则需进行rTMS再治疗直到恢复至之前的缓解水平,此类患者可考虑采用随时间递减或密集式rTMS维持治疗方案来预防抑郁复发。
3 rTMS联合药物治疗
rTMS与抗抑郁药物联合使用是常见的治疗方案,该联合应用策略目前存在“疗效等同”和“附加增效”2种结论。先前的大型、多中心RCT研究比较了右侧LF-rTMS与文拉法辛的抗抑郁效果发现,单独使用LF-rTMS、单独使用文拉法辛以及二者联合的疗效相当[69]。但近年来多数研究支持rTMS在加速MDD患者对抗抑郁药物反应方面的“附加效应”,即rTMS与抗抑郁药(选择性5-羟色胺再摄取抑制剂)结合使用可能比单独使用rTMS 效果更佳,具有更强的抗抑郁效果[45-46]。
在人群特征方面,研究一致认为,HF-rTMS 联合抗抑郁药治疗比单用药物可更迅速地改善首次发作的年轻MDD患者的抑郁症状;此外,该疗法的可接受性和安全性与伪刺激治疗联合抗抑郁药相当[70]。长期随访研究表明,无论是作为单一疗法还是附加疗法,rTMS在预防抑郁复发方面均优于抗抑郁药,且不增加躁狂风险[65]。基于现有的研究,国际临床神经生理学联合会修订建议时指出,单独使用rTMS治疗与联合使用抗抑郁药物在抗抑郁疗效上可能不存在显著差异[18]。即便如此,目前并不能完全否定rTMS联合药物的抗抑郁潜力,这种附加效应可能受药物类别(选择性5-羟色胺再摄取抑制剂)、疾病阶段(首次发作)和人群特征(年轻患者)三重影响。
4 新型 rTMS治疗方案
尽管rTMS在治疗MDD方面已取得一定进展,其效率和效果仍存在较大提升空间。近年来,研究者们开发了多种新型rTMS治疗方案,以缩短治疗时间、增强抗抑郁效果。
为增强抗抑郁效果并缩短治疗周期,有研究者提出了每日多次治疗的“加速rTMS方案”[71]。研究显示,加速 rTMS 方案对MDD患者(包括老年患者群体)均具有良好的安全性和耐受性[72]。为进一步提高治疗效率,有研究者开发了θ爆发式刺激(TBS)。与传统HF/LF-rTMS方案不同,TBS模拟内源性大脑θ节律,以固定的脉冲程序发放更低强度的快速刺激。间歇性TBS可在短短3 min内释放600脉冲,其对大脑皮层的兴奋性效应与常规HF-rTMS刺激相似甚至更强。Blumberger等[73]于2018年进行的一项大规模非劣效性研究表明,左侧前额叶间歇性TBS 在减少抑郁症状、提高响应率和实现症状缓解方面均不逊色于HF-rTMS,二者在耐受性和安全性方面也无显著差异;而每次治疗时间却从37.5 min大幅下降至3 min,从而极大提高了rTMS的治疗效率。
此外,越来越多的研究发现,DLPFC作为刺激靶点存在显著个体差异,可能影响个体对治疗的反应[74]。因此,对于刺激靶点的定位逐步从传统的基于头皮测量(如“5 cm法”或“F3法”)的定位方式向基于神经影像(如结构性/功能性磁共振)的个体化定位转变。Cole等[75-76]通过靶向刺激个体DLPFC内与膝下前扣带回皮质(sgACC)功能连接负相关最强的区域,并结合加速间歇性TBS 方案(每天治疗10次,连续5 d),开发了“斯坦福神经调节疗法”(SAINT),显著提高了rTMS的抗抑郁效果。但也有研究者持反对观点,认为左侧DLPFC与sgACC的负向功能连接不能作为rTMS抗抑郁反应的生物标志物[77]。另外,功能磁共振的不稳定性也使个体化靶点定位的可重复性受到挑战。因此,未来需开展更多前瞻性RCT研究以评估基于连接性的rTMS个体化靶向方法相较于传统方法的优越性。
5 小结与展望
rTMS作为一种无创神经调控技术,被广泛用作抑郁发作患者的急性治疗手段,维持治疗也展现出预防抑郁复发的潜力(图1)。

图1 rTMS在抑郁障碍治疗中的应用
rTMS:同表1
然而,目前针对rTMS急性期疗效的预测标志仍不明确,维持治疗方案也缺乏共识。未来研究应聚焦于深入揭示rTMS的作用机制,并基于患者特征优化治疗参数,以提供更个性化、更高效的治疗方案。此外,现有的rTMS设备体积较大,操作复杂,需由专业人员在医疗机构内使用,这显著增加了患者的时间和经济成本,也是长期维持治疗研究中患者脱落率较高的原因之一。可在家庭环境使用的便携式rTMS设备的研发有望显著提高治疗的可及性和依从性,在MDD的维持治疗中发挥积极作用。
参考文献
[1]Lu J, Xu X F, Huang Y Q, et al. Prevalence of depressive disorders and treatment in China: a cross-pal epidemiological study[J]. Lancet Psychiatry, 2021, 8(11): 981-990.
[2]Malhi G S, Mann J J. Depression[J]. Lancet, 2018, 392(10161): 2299-2312.
[3]Rush A J, Trivedi M H, Wisniewski S R, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report[J]. Am J Psychiatry, 2006, 163(11): 1905-1917.
[4]Monroe S M, Harkness K L. Recurrence in major depression: a conceptual analysis[J]. Psychol Rev, 2011, 118(4): 655-674.
[5]Rybak Y E, Lai K S P, Ramasubbu R, et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment[J]. Depress Anxiety, 2021, 38(4): 456-467.
[6]O'Reardon J P, Solvason H B, Janicak P G, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial[J]. Biol Psychiatry, 2010, 67(2): 1208-1216.
[7]Milev R V, Giacobbe P, Kennedy S H, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: p 4. Neurostimulation treatments[J]. Can J Psychiatry, 2016, 61(9): 561-575.
[8]Fitzgerald P B, Hoy K E, Anderson R J, et al. A study of the pattern of response to rTMS treatment in depression[J]. Depress Anxiety, 2016, 33(8): 746-753.
[9]George M S. Whither TMS: a one-trick pony or the beginning of a neuroscientific revolution?[J]. Am J Psychiatry, 2019, 176(11): 904-910.
[10]Lisanby S H. Noninvasive brain stimulation for depression-the devil is in the dosing[J]. N Engl J Med, 2017, 376(26): 2593-2594.
[11]Mayberg H S, Brannan S K, Tekell J L, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response[J]. Biol Psychiatry, 2000, 48(8): 830-843.
[12]Brunoni A R, Chaimani A, Moffa A H, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis[J]. JAMA Psychiatry, 2017, 74(2): 143-152.
[13]Sehatzadeh S, Daskalakis Z J, Yap B, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: a meta-analysis of randomized controlled trials over 2 decades[J]. J Psychiatry Neurosci, 2019, 44(3): 151-163.
[14]Croarkin P E, Nakonezny P A, Deng Z D, et al. High-frequency repetitive TMS for suicidal ideation in adolescents with depression[J]. J Affect Disord, 2018, 239: 282-290.
[15]Janicak P G, Dunner D L, Aaronson S T, et al. Transcr-anial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of quality of life outcome measures in clinical practice[J]. CNS Spectr, 2013, 18(6): 322-332.
[16]Wang W L, Wang S Y, Hung H Y, et al. Safety of transcranial magnetic stimulation in unipolar depression: a systematic review and meta-analysis of randomized-controlled trials[J]. J Affect Disord, 2022, 301: 400-425.
[17]Miron J P, Jodoin V D, Lespérance P, et al. Repetitive transcranial magnetic stimulation for major depressive disorder: basic principles and future directions[J]. Ther Adv Psychopharmacol, 2021, 11: 20451253211042696.
[18]Lefaucheur J P, Aleman A, Baeken C, et al. Evidencebased guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014—2018)[J]. Clin Neurophysiol, 2020, 131(2): 474-528.
[19]Hussain S, Chamoli S, Fitzgerald P, et al. Royal Australian and New Zealand college of psychiatrists professional practice guidelines for the administration of repetitive transcranial magnetic stimulation[J]. Aust N Z J Psychiatry, 2024, 58(8): 641-655.
[20]Berlow Y A, Zandvakili A, Philip N S. Low frequency right-sided and high frequency left-sided repetitive transcranial magnetic stimulation for depression: the evidence of equivalence[J]. Brain Stimul, 2020, 13(6): 1793-1795.
[21]Aaronson S T, Carpenter L L, Hutton T M, et al. Compari-son of clinical outcomes with left unilateral and sequential bilateral Transcranial Magnetic Stimulation (TMS) treatment of major depressive disorder in a large patient registry[J]. Brain Stimul, 2022, 15(2): 326-336.
[22]Berlim M T, Van Den Eynde F, Daskalakis Z J. Efficacy and acceptability of high frequency repetitive transcranial magnetic stimulation (rTMS) versus electroconvulsive therapy (ECT) for major depression: a systematic review and meta-analysis of randomized trials[J]. Depress Anxiety, 2013, 30(7): 614-623.
[23]Chen L, Hudaib A R, Hoy K E, et al. Is rTMS effective for anxiety symptoms in major depressive disorder? An efficacy analysis comparing left-sided high-frequency, right-sided low-frequency, and sequential bilateral rTMS protocols[J]. Depress Anxiety, 2019, 36(8): 723-731.
[24]Fitzgerald P B, Hoy K E, Reynolds J, et al. A pragmatic randomized controlled trial exploring the relationship between pulse number and response to repetitive transcranial magnetic stimulation treatment in depression[J]. Brain Stimul, 2020, 13(1): 145-152.
[25]McCalley D M, Lench D H, Doolittle J D, et al. Determin-ing the optimal pulse number for theta burst induced change in cortical excitability[J]. Sci Rep, 2021, 11(1): 8726.
[26]Teng S, Guo Z, Peng H, et al. High-frequency repetitive transcranial magnetic stimulation over the left DLPFC for major depression: session-dependent efficacy: a meta-analysis[J]. Eur Psychiatry, 2017, 41: 75-84.
[27]Hutton T M, Aaronson S T, Carpenter L L, et al. Dosing transcranial magnetic stimulation in major depressive disorder: relations between number of treatment sessions and effectiveness in a large patient registry[J]. Brain Stimul, 2023, 16(5): 1510-1521.
[28]Yu T, Chen W N, Huo L J, et al. Association between daily dose and efficacy of rTMS over the left dorsolateral prefrontal cortex in depression: a meta-analysis[J]. Psychiatry Res, 2023, 325: 115260.
[29]Dalhuisen I, Van Bronswijk S, Bors J, et al. The association between sample and treatment characteristics and the efficacy of repetitive transcranial magnetic stimulation in depression: a meta-analysis and meta-regression of sham-controlled trials[J]. Neurosci Biobehav Rev, 2022, 141: 104848.
[30]Hsu T W, Yeh T C, Kao Y C, et al. The dose-effect relationship of six stimulation parameters with rTMS over left DLPFC on treatment-resistant depression: a systematic review and meta-analysis[J]. Neurosci Biobehav Rev, 2024, 162: 105704.
[31]McClintock S M, Reti I M, Carpenter L L, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression[J]. J Clin Psychiatry, 2018, 79(1): 16cs10905.
[32]Sigrist C, Vöckel J, MacMaster F P, et al. Transcranial magnetic stimulation in the treatment of adolescent depression: a systematic review and meta-analysis of aggregated and individual-patient data from uncontrolled studies[J]. Eur Child Adolesc Psychiatry, 2022, 31(10): 1501-1525.
[33]Qiao Y, Wang J J, Zhu J J, et al. Antidepressant effect of adjunct repetitive transcranial magnetic stimulation in inpatients 60 years and older[J]. J ECT, 2020, 36(3): 216-221.
[34]Sackeim H A, Aaronson S T, Carpenter L L, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation[J]. J Affect Disord, 2020, 277: 65-74.
[35]Cotovio G, Boes A D, Press D Z, et al. In older adults the antidepressant effect of repetitive transcranial magnetic stimulation is similar but occurs later than in younger adults[J]. Front Aging Neurosci, 2022, 14: 919734.
[36]Clarke E, Clarke P, Gill S, et al. Efficacy of repetitive transcranial magnetic stimulation in the treatment of depression with comorbid anxiety disorders[J]. J Affect Disord, 2019, 252: 435-439.
[37]Philip N S, Leuchter A F, Cook I A, et al. Predictors of response to synchronized transcranial magnetic stimulation for major depressive disorder[J]. Depress Anxiety, 2019, 36(3): 278-285.
[38]Carpenter L L, Conelea C, Tyrka A R, et al. 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder[J]. J Affect Disord, 2018, 235: 414-420.
[39]Yesavage J A, Fairchild J K, Mi Z B, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial[J]. JAMA Psychiatry, 2018, 75(9): 884-893.
[40]Madore M R, Kozel F A, Williams L M, et al. Prefrontal transcranial magnetic stimulation for depression in US military veterans-A naturalistic cohort study in the veterans health administration[J]. J Affect Disord, 2022, 297: 671-678.
[41]Thatikonda N S, Vinod P, Balachander S, et al. Efficacy of repetitive transcranial magnetic stimulation on comorbid anxiety and depression symptoms in obsessive-compulsive disorder: a meta-analysis of randomized sham-controlled trials[J]. Can J Psychiatry, 2023, 68(6): 407-417.
[42]Ward H B, Yip A, Siddiqui R, et al. Borderline personality traits do not influence response to TMS[J]. J Affect Disord, 2021, 281: 834-838.
[43]Trapp N T, Purgianto A, Taylor J J, et al. Consensus review and considerations on TMS to treat depression: a comprehensive update endorsed by the National Network of Depression Centers, the Clinical TMS Society, and the International Federation of Clinical Neurophysiology[J]. Clin Neurophysiol, 2025, 170: 206-233.
[44]Lisanby S H, Husain M M, Rosenquist P B, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial[J]. Neuropsychopharmacology, 2009, 34(2): 522-534.
[45]Hung Y Y, Yang L H, Stubbs B, et al. Efficacy and tolerability of deep transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2020, 99: 109850.
[46]Zaidi A, Shami R, Sewell I J, et al. Antidepressant class and concurrent rTMS outcomes in major depressive disorder: a systematic review and meta-analysis[J]. EClinicalMedicine, 2024, 75: 102760.
[47]Deppe M, Abdelnaim M, Hebel T, et al. Concomitant lorazepam use and antidepressive efficacy of repetitive transcranial magnetic stimulation in a naturalistic setting[J]. Eur Arch Psychiatry Clin Neurosci, 2021, 271(1): 61-67.
[48]Hunter A M, Minzenberg M J, Cook I A, et al. Concomitant medication use and clinical outcome of repetitive Transcr-anial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder[J]. Brain Behav, 2019, 9(5): e01275.
[49]Van Eijndhoven P F P, Bartholomeus J, Möbius M, et al. A randomized controlled trial of a standard 4-week protocol of repetitive transcranial magnetic stimulation in severe treatment resistant depression[J]. J Affect Disord, 2020, 274: 444-449.
[50]Kedzior K K, Reitz S K, Azorina V, et al. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) In the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials[J]. Depress Anxiety, 2015, 32(3): 193-203.
[51]Hsu T W, Yeh T C, Kao Y C, et al. Response trajectory to left dorsolateral prefrontal rTMS in major depressive disorder: a systematic review and meta-analysis: trajectory of rTMS[J]. Psychiatry Res, 2024, 338: 115979.
[52]Senova S, Cotovio G, Pascual-Leone A, et al. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis[J]. Brain Stimul, 2019, 12(1): 119-128.
[53]Cohen R B, Boggio P S, Fregni F. Risk factors for relapse after remission with repetitive transcranial magnetic stimula-tion for the treatment of depression[J]. Depress Anxiety, 2009, 26(7): 682-688.
[54]Demirtas-Tatlidede A, Mechanic-Hamilton D, Press D Z, et al. An open-label, prospective study of repetitive transcranial magnetic stimulation (rTMS) in the long-term treatment of refractory depression: reproducibility and duration of the antidepressant effect in medication-free patients[J]. J Clin Psychiatry, 2008, 69(6): 930-934.
[55]Miljevic A, Bailey N W, Herring S E, et al. Potential predictors of depressive relapse following repetitive Transcranial Magnetic Stimulation: a systematic review[J]. J Affect Disord, 2019, 256: 317-323.
[56]Richieri R, Guedj E, Michel P, et al. Maintenance transcranial magnetic stimulation reduces depression relapse: a propensity-adjusted analysis[J]. J Affect Disord, 2013, 151(1): 129-135.
[57]Rosenberg O, Dinur Klein L, Gersner R, et al. Long-term follow-up of MDD patients who respond to deep rTMS: a brief report[J]. Isr J Psychiatry Relat Sci, 2015, 52(1): 17-23.
[58]Wilson S, Croarkin P E, Aaronson S T, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS[J]. J Affect Disord, 2022, 296: 79-88.
[59]Fitzgerald P B, Grace N, Hoy K E, et al. An open label trial of clustered maintenance rTMS for patients with refractory depression[J]. Brain Stimul, 2013, 6(3): 292-297.
[60]Brian Chen Y C, Chou P H, Tu Y K, et al. Trajectory of changes in depressive symptoms after acute repetitive transcranial magnetic stimulation: a meta-analysis of follow-up effects[J]. Asian J Psychiatr, 2023, 88: 103717.
[61]Benadhira R, Thomas F, Bouaziz N, et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD)[J]. Psychiatry Res, 2017, 258: 226-233.
[62]Philip N S, Dunner D L, Dowd S M, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study[J]. Brain Stimul, 2016, 9(2): 251-257.
[63]Rapinesi C, Bersani F S, Kotzalidis G D, et al. Maintenance deep transcranial magnetic stimulation sessions are associated with reduced depressive relapses in patients with unipolar or bipolar depression[J]. Front Neurol, 2015, 6: 16.
[64]Matsuda Y, Sakuma K, Kishi T, et al. Repetitive transcranial magnetic stimulation for preventing relapse in antidepressant treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials[J]. Brain Stimul, 2023, 16(2): 458-461.
[65]Wang H N, Wang X X, Zhang R G, et al. Clustered repetitive transcranial magnetic stimulation for the prevention of depressive relapse/recurrence: a randomized controlled trial[J]. Transl Psychiatry, 2017, 7(12): 1292.
[66] 许毅, 李达, 谭立文, 等. 重复经颅磁刺激治疗专家共识[J]. 转化医学杂志, 2018, 7(1): 4-9.
[67] Perera T, George M S, Grammer G, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder[J]. Brain Stimul, 2016, 9(3): 336-346.
[68]Kelly M S, Bernstein M, Oliveira-Maia A J, et al. 15-Response to first course of TMS treatment for depression predicts subsequent response[J]. Brain Stimul, 2016, 9(5): e5.
[69]Brunelin J, Jalenques I, Trojak B, et al. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT[J]. Brain Stimul, 2014, 7(6): 855-863.
[70]Cao P P, Li Y H, An B, et al. Efficacy and safety of repetitive transcranial magnetic stimulation combined with antidepressants in children and adolescents with depression: a systematic review and meta-analysis[J]. J Affect Disord, 2023, 336: 25-34.
[71]McGirr A, Van Den Eynde F, Tovar-Perdomo S, et al. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: an open label trial[J]. J Affect Disord, 2015, 173: 216-220.
[72]Dardenne A, Baeken C, Crunelle C L, et al. Accelerated HF-rTMS in the elderly depressed: a feasibility study[J]. Brain Stimul, 2018, 11(1): 247-248.
[73]Blumberger D M, Vila-Rodriguez F, Thorpe K E, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial[J]. Lancet, 2018, 391(10131): 1683-1692.
[74]Cash R F H, Weigand A, Zalesky A, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression[J]. Biol Psychiatry, 2021, 90(10): 689-700.
[75]Cole E J, Phillips A L, Bentzley B S, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial[J]. Am J Psychiatry, 2022, 179(2): 132-141.
[76]Cole E J, Stimpson K H, Bentzley B S, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression[J]. Am J Psychiatry, 2020, 177(8): 716-726.
[77]Hopman H J, Chan S M S, Chu W C W, et al. Personalized prediction of transcranial magnetic stimulation clinical response in patients with treatment-refractory depression using neuroimaging biomarkers and machine learning[J]. J Affect Disord, 2021, 290: 261-271.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)