首页 > 医疗资讯/ 正文

研究背景
低血压是剖宫产椎管内麻醉时的常见并发症,长期低血压可导致产妇恶心呕吐或引起胎儿发生酸中毒,应预防性使用血管升压药预防脊麻性低血压的发生。脊麻性低血压定义为从椎管内麻醉到分娩的过程中,收缩压降至基础值的80 %以下或< 90mmHg,重度脊麻性低血压定义为收缩压降至基础值的60 %以下。研究表明去甲肾上腺素可以用于替代去氧肾上腺素,与单纯的α-1肾上腺素能受体激动剂去氧肾上腺素相比,去甲肾上腺素通过其较弱的β-肾上腺素能受体激动活性引起较小的压力感受性心率抑制。既往研究发现,当结合去甲肾上腺素首次静脉推注预防剖宫产术中脊麻性低血压时,0.05μg/kg/min的去甲肾上腺素持续输注较合理;当去甲肾上腺素持续输注速率为0.05μg/kg/min时,给予起始剂量0.10μg/kg去甲肾上腺素较能更有效地降低脊麻性低血压的发生率。本研究拟以0.05μg/kg/min的速率持续输注去甲肾上腺素,同时给予安慰剂或3种基于体重的初始剂量(0.05μg/kg、0.10μg/kg、0.15μg/kg),旨在探索去甲肾上腺素输注剂量与剖宫产术中脊麻性低血压发生率之间的剂量反应关系,同时评估输注不同剂量去甲肾上腺素时的不良产妇或新生儿反应,并确定当连续输注速率为0.05μg/kg/min时,预防椎管内低血压所需的去甲肾上腺素最佳首次剂量。
研究方法
本研究纳入120例择期剖宫产单胎妊娠产妇。纳入标准为:年龄在18-40岁之间,足月(≥37孕周)。排除标准为:高血压、心血管疾病、基础收缩压< 100 mm Hg、身高<145cm或> 180cm、体重<40kg或>100kg、凝血功能异常和胎儿畸形。经蛛网膜下腔阻滞后产妇被随机分为四组:0组、0.05组、0.10组、0.15组,分别给予去甲肾上腺素0μg/kg 、0.05μg/kg 、0.10μg/kg 、0.15μg/kg ,继之以0.05μg/kg/min的速率持续输注去甲肾上腺素。
入室后,对产妇进行常规监测,右臂用于监测血压,患者仰卧位测得血压基线值。患者取右侧卧位,于L3-4水平注入0.5%等比重罗哌卡因3mL行蛛网膜下腔阻滞。随后,患者取仰卧位左侧倾斜体位。于手术切皮前将患者恢复至全仰卧位,评估感觉阻滞平面,感觉阻滞平面低于T6水平的产妇被排除。
开始椎管内麻醉后,立即快速静注初始剂量的去甲肾上腺素,手术过程中持续输注给药,直至胎儿产出后5min。若产妇出现低血压,给予去甲肾上腺素8μg,若心率低于55次/分,给予阿托品0.5mg,若收缩压较基线值升高>20%,则停用去甲肾上腺素,若脉搏血氧饱和度<95 %,则使用面罩吸氧。林格氏液从进入手术室到胎儿娩出的输注量高达1.5L。输注去甲肾上腺素时将林格氏液的输注速度调整为20mL/min。缩宫素在胎儿产出后以100μg缓慢静脉注射给药。
结果采用Probit分析估计 ED90和ED95。主要结果为脊麻性低血压的发生率,次要结果为与产妇、胎儿和血流动力学改变有关的其他结果,产妇结局包括严重的脊麻性低血压、高血压、心动过缓和恶心和/或呕吐的发生率。同时记录去甲肾上腺素和阿托品的额外给药剂量,胎儿结局通过Apgar评分和脐动脉血气分析进行评估。
结果
共有132名产妇符合纳入标准,其中12名被排除,共有120名产妇被随机分为四组。在120例受试者中,有3例患者因蛛网膜下腔阻滞失败而未接受分配。最终117名患者接受评估。
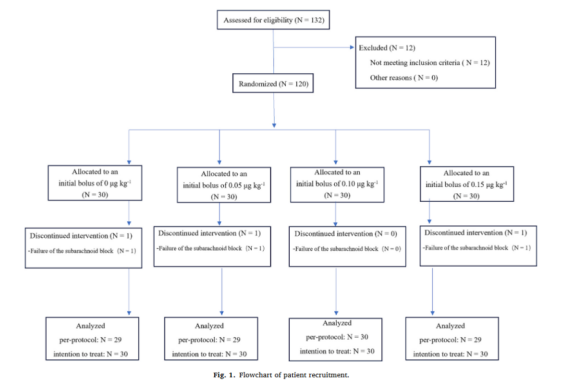
图1:患者招募流程图
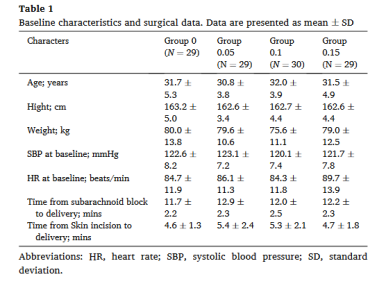
表1:基线特征和手术资料
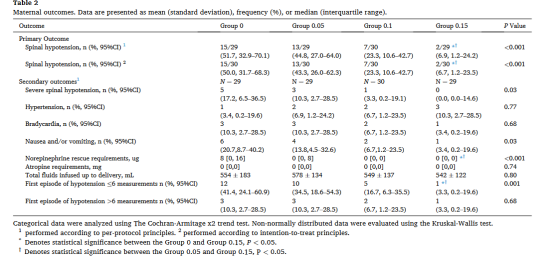
主要结局:根据符合方案分析,脊麻性低血压的发生率在各组间差异显著(P<0.001):0组51.7%;0.05组,44.8%;0.10组,23.3 %;0.15组,6.9%(表2)。剂量反应曲线见图2。去甲肾上腺素的ED90和ED95分别为0.150μg/kg和0.187μg/kg。根据意向性分析原则,脊麻性低血压的发生率在各组间差异显著(P<0.001):0组:50.0 %;0.05组:43.3%;0.10组:23.3%;0.15组:6.7%(表2)。ED90和ED95分别为0.150μg/kg和0.188μg/kg。
表2:产妇结局
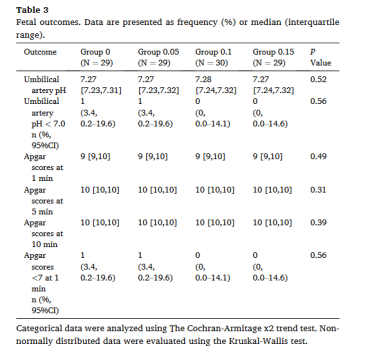
次要结局:各组重度脊麻性低血压发生率、去甲肾上腺素的额外给药剂量、恶心和/或呕吐的发生率存在显著差异(表2);各组心动过缓和高血压的发生率无显著差异(表2);各组间的Apgar评分和脐动脉血pH无显著差异(表3)。
表3:妊娠结局
收缩压在前十次测量中的变化见图3,各组之间曲线下面积(平均值±标准差)存在显著差异。
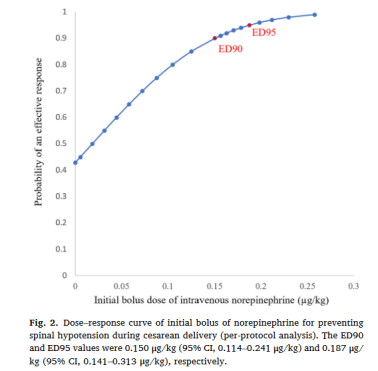
图2:去甲肾上腺素预防剖宫产脊麻性低血压初始剂量-反应曲线
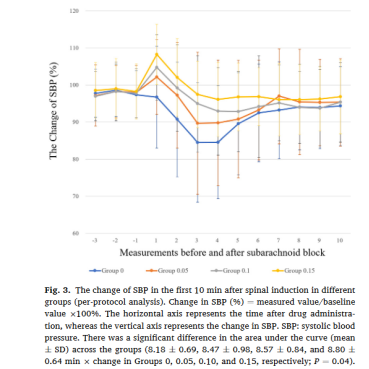
图3:各组脊麻诱导后10min收缩压变化
讨论
本研究确定了从椎管内麻醉开始到胎儿产出过程中初始静推去甲肾上腺素预防脊麻性低血压的剂量-反应关系。去甲肾上腺素首次静推0.15μg/kg,结合持续输注速率0.05μg/kg/min,可降低椎管内低血压的发生率,减少去甲肾上腺素的额外给药剂量。在常规血压监测下,应根据低血压的严重程度调整输注速度。
与以往研究不同,本研究采用的是以体重为基础的推注给药方式,而不是固定剂量给药。在既往研究中,治疗脊麻性低血压的去甲肾上腺素用量为12.3μg,相较于预防所需剂量更大。鉴于去甲肾上腺素的半衰期较短,因此需通过持续输注去甲肾上腺素来维持其血浆浓度的稳定。值得注意的是,最近关于预防性静注去甲肾上腺素预防腰麻下择期剖宫产术中低血压的meta分析确定了0.2μg/kg/min为去甲肾上腺素持续输注速率的ED95。与上述研究相比,本研究使用了更低的去甲肾上腺素剂量,可以达到相同的预防效果。随着输注剂量的增加,低血压的发生率降低,而高血压的发生率显著增加。因此,单独采用去甲肾上腺素持续输注的方式可能无法达到最佳效果,而单次推注给药可减小所需的持续输注速率,预防脊麻性低血压发生的效果更佳。
本研究选择0.05μg/kg/min的原因有以下几点:首先,先前的研究没有涉及持续输注过程中的推注给药。有研究表明,与给予去甲肾上腺素0.050μg/kg/ min相比,先给予去甲肾上腺素5μg,再给予去甲肾上腺素0.075μg/kg/min,并不能降低低血压的发生率。因此,0.050μg/kg/min为蛛网膜下腔阻滞后合理的输注剂量。第二,反应性高血压的发生率可能随着去甲肾上腺素输注剂量的增加而增加。有研究表明,当去甲肾上腺素输注剂量为0.1μg/kg/min时,腰麻后高血压的发生率为35%,而当去甲肾上腺素输注剂量降至0.05μg/kg/min时,腰麻后高血压的发生率降至10%。其次,已有研究表明,以0.05μg/kg/min的速率输注去甲肾上腺素可有效降低脊麻性低血压的发生率。最后,有研究表明,当去甲肾上腺素输注速率为0.05μg/kg/min时,首次推注0.10μg/kg的去甲肾上腺素比0.05μg/kg能更有效地降低脊髓低血压。
局限性:由于动脉穿刺可能引起患者疼痛,影响基础血压的测定,故未对心输出量进行监测,首次推注去甲肾上腺素可能增加心输出量或体循环血管阻力,这一点可以在进一步的研究中得到证实。手术开始后产妇取仰卧位,与左侧卧位相比,仰卧位需要更高剂量的去氧肾上腺素,但子宫动脉超负荷量组间差异无统计学意义。此外,尽管一些研究认为0.05μg/kg/min的输注速率比较合理,但预防剖宫产术中脊麻性低血压的最佳推注剂量尚未得出。在改变输注量或椎管内用药剂量后,可能需要重新计算血容量,因此仍需进一步研究。最后,首次给药剂量的ED95在我们的研究剂量范围之外,仍需要进一步的研究来验证其对剖宫产术中脊麻性低血压的影响。
综上所述,当首次推注剂量为0.150μg/kg,去甲肾上腺素持续输注速率为0.05μg / kg / min时,预防剖宫产术中脊麻性低血压最有效。这种给药方案显著降低了产妇出现恶心和呕吐的风险,同时并未显著提高高血压的发生率。
产麻新谭·点评
剖宫产术中产妇经常发生恶心呕吐,脊麻性低血压可导致脑干缺血和呕吐中枢的激活,还会导致内脏血流量减少,引起胃肠道释放催吐因子,因此减少低血压的发生可显著降低术中恶心和呕吐的发生率。以往研究表明,以0.05μg/kg/min的速度输注去甲肾上腺素并结合首次静脉推注可降低剖宫产术中脊麻性低血压的发生率。初始去甲肾上腺素推注量影响持续输注时脊麻性低血压的发生,但持续输注速率为0.05μg/kg/min时的理想初始剂量尚不清楚。本研究将120例择期剖宫产产妇随机分为4组,分别给予0、0.05、0.10和0.15μg/kg的去甲肾上腺素,然后以0.05μg/kg/min的速度持续输注。主要结局指标为初始去甲肾上腺素推注量在预防脊麻性低血压发生中的剂量反应关系。次要结局指标包括恶心和/或呕吐、高血压、心动过缓的发生率,以及Apgar评分和脐动脉血气分析结果。ED90和ED95采用probit回归进行估计。探索发现首次静推0.150μg/kg去甲肾上腺素可能是持续输注速率为0.05μg/kg/min时预防剖宫产脊麻性低血压的最佳剂量,同时未明显增加高血压的发生率,但可显著降低发生恶心和/或呕吐的风险。这为优化去甲肾上腺素的给药方案以及减少脊麻性低血压所致的术中恶心呕吐等一系列并发症提出了可行性的建议。
参考文献
1. Sakata K, Yoshimura N, Tanabe K, Kito K, Nagase K, Iida H. Prediction of hypotension during spinal anesthesia for elective cesarean p by altered heart rate variability induced by postural change. Int J Obstet Anesth 2017;29:34–8.
2. Kuhn JC, Haμge TH, Rosseland LA, Dahl V, Langesæter E. Hemodynamics of phenylephrine infusion versus lower extremity compression during spinal anesthesia for cesarean delivery: a randomized, double-blind, placebo-controlled study. Anesth Analg 2016;122:1120–9.
3. Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean p under spinal anaesthesia. Anaesthesia 2018;73:71–92.
4. Ngan Kee WD. Norepinephrine for maintaining blood pressure during spinal anaesthesia for caesarean p: a 12-month review of individual use. Int J Obstet Anesth 2017;30:73–4.
5. Ngan Kee WD, Lee SWY, Ng FF, Khaw KS. Prophylactic norepinephrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 2018;126:1989–94.
6. Vallejo MC, Attaallah AF, Elzamzamy OM, et al. An open-label randomized controlled clinical trial for comparison of continuous phenylephrine versus norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth 2017;29:18–25.
7. Ngan Kee WD, Lee SW, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology 2015;122: 736–45.
8. Ngan Kee WD, Lee SWY, Ng FF, Lee A. Norepinephrine or phenylephrine during spinal anaesthesia for caesarean delivery: a randomised double-blind pragmatic non-inferiority study of neonatal outcome. Br J Anaesth 2020;125:588–95.
9. Rai AV, Prakash S, Chellani H, Mullick P, Wason R. Comparison of phenylephrine and norepinephrine for treatment of spinal hypotension during elective cesarean delivery- a randomised, double-blind study. J Anaesthesiol Clin Pharmacol 2022; 38:445–52.
10. Hasanin AM, Amin SM, Agiza NA, et al. Norepinephrine infusion for preventing Postspinal anesthesia hypotension during cesarean delivery: a randomized dose finding trial. Anesthesiology 2019;130(1):55–62.
11. Chen Y, Zou L, Li Z, et al. Prophylactic norepinephrine infusion for postspinal anesthesia hypotension in patients undergoing cesarean p: a randomized, controlled, dose-finding trial. Pharmacotherapy 2021;41(4):370–8.
12. Lyu W, Wei P, Tang W, et al. Preventing spinal hypotension during cesarean birth with two initial boluses of norepinephrine in Chinese Parturients: a randomized, double-blind. Controll Trial Anesth Analg 2023;136:94–100.
13. Bishop DG, Cairns C, Grobbelaar M, Rodseth RN. Prophylactic phenylephrine infusions to reduce severe spinal anesthesia hypotension during cesarean delivery in a resource-constrained environment. Anesth Analg 2017;125(3):904–6.
14. Qian J, Zhao YP, Deng JL, et al. Determination of the relative potency of norepinephrine and phenylephrine given as infusions for preventing hypotension during combined spinal-epidural anesthesia for cesarean delivery: a randomized up-and-down sequential allocation study. Front Pharmacol 2022;13:942005.
15. Singh J, Singh J, Mitra S, Anand LK, Goel B, Kaur M. Comparison of prophylactic phenylephrine and norepinephrine infusion on umbilical arterial pH and maternal blood pressure during spinal anaesthesia for caesarean delivery. Indian J Anaesth 2022;66(Suppl. 2):S115–21.
16. Guo L, Xu X, Qin R, Shi Y, Xue W, He L, et al. Prophylactic norepinephrine and phenylephrine boluses to prevent Postspinal anesthesia hypotension during cesarean p: a randomized sequential allocation dose-finding study. Drug Des Devel Ther 2023;17:1547–55.
17. Sheng ZM, Shen YP, Pan ZB, et al. Comparative study on the manually-controlled variable-rate versus fixed-rate infusion of norepinephrine for preventing hypotension during spinal anesthesia for cesarean delivery. J Clin Anesth 2022;82: 110944.
18. Sheng Z, Sun H, Liu J, Qian X. Comparative dose–response study on
norepinephrine infusion for preventing hypotension during spinal anaesthesia for caesarean delivery in singleton versus twin pregnancies: A randomized, double blind, controlled, dose-finding trial. Eur J Anaesthesiol 2021;38(8):895–7.
19. Chen D, Qi X, Huang X, et al. Efficacy and safety of different norepinephrine regimens for prevention of spinal hypotension in cesarean p: a randomized trial. Biomed Res Int 2018;2018:2708175.
20. Onwochei DN, Ngan Kee WD, Fung L, Downey K, Ye XY, Carvalho JCA. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg 2017;125:212–8.
21. Xu T, Zheng J, An XH, Xu ZF, Wang F. Norepinephrine intravenous prophylactic bolus versus rescue bolus to prevent and treat maternal hypotension after combined spinal and epidural anesthesia during cesarean delivery: a sequential dose-finding study. Ann Transl Med 2019;7(18):451.
22. Xu W, Drzymalski DM, Ai L, Yao H, Liu L, Xiao F. The ED50 and ED95 of prophylactic norepinephrine for preventing post-spinal hypotension during cesarean delivery under combined spinal-epidural anesthesia: a prospective dose-finding study. Front Pharmacol 2021;12:691809.
23. Li Y, Shuai B, Huang H. Prophylactic intravenous norepinephrine for the prevention of hypotension during spinal anesthesia for elective cesarean p: a systematic review and dose-response meta-analysis of randomized controlled trials. Front Pharmacol 2023;14:1247214.
24. Fu F, Xiao F, Chen W, et al. A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for caesarean delivery. Br J Anaesth 2020; 124(3):e108–14.
25. Wei C, Qian J, Zhang Y, Chang X, Hu H, Xiao F. Norepinephrine for the prevention of spinal-induced hypotension during caesarean delivery under combined spinal-epidural anaesthesia: randomised, double-blind, dose-finding study. Eur J Anaesthesiol 2020;37:309–15.
26. Baytas¸ V, Karadag˘ Erkoç S, Ozçelik ¨ M, et al. A randomized, double-blind, graded dose-response study of norepinephrine Administration for Prevention of post-spinal hypotension during elective cesarean delivery. J Clin Med 2023;12(20):6437.
27. Chen Y, Guo L, Shi Y, et al. Norepinephrine prophylaxis for postspinal anesthesia hypotension in parturient undergoing cesarean p: a randomized, controlled trial. Arch Gynecol Obstet 2020;302(4):829–36.
28. Hasanin A, Amin S, Refaat S, et al. Norepinephrine versus phenylephrine infusion for prophylaxis against post-spinal anaesthesia hypotension during elective caesarean delivery: a randomised controlled trial. Anaesth Crit Care Pain Med 2019;38:601–7.
29. Carvalho B, Cohen SE, Lipman SS, Fuller A, Mathusamy AD, Macario A. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg 2005;101:1182–7.
30. Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean p under regional anesthesia. Int J Obstet Anesth 2005;14:230–41.
31. Cooperman LH. Effects of anaesthetics on the splanchnic circulation. Br J Anaesth 1972;44:967–70.
32. Manouchehrian N, Moradi A, Torkashvand L. Comparative study of effect of spinal anesthesia in sitting and lateral positions on the onset time of sensory block and hemodynamic condition in cesarean p: a randomized clinical trial. Anesth Pain Med 2021;11(1):e111483.
33. Lee AJ, Landau R, Mattingly JL, et al. Left lateral table tilt for elective cesarean delivery under spinal anesthesia has no effect on neonatal Acid-Base status: a randomized controlled trial. Anesthesiology 2017;127:241–9.
- 搜索
-
- 1000℃李寰:先心病肺动脉高压能根治吗?
- 1000℃除了吃药,骨质疏松还能如何治疗?
- 1000℃抱孩子谁不会呢?保护脊柱的抱孩子姿势了解一下
- 1000℃妇科检查有哪些项目?
- 1000℃妇科检查前应做哪些准备?
- 1000℃女性莫名烦躁—不好惹的黄体期
- 1000℃会影响患者智力的癫痫病
- 1000℃治女性盆腔炎的费用是多少?
- 标签列表
-
- 星座 (702)
- 孩子 (526)
- 恋爱 (505)
- 婴儿车 (390)
- 宝宝 (328)
- 狮子座 (313)
- 金牛座 (313)
- 摩羯座 (302)
- 白羊座 (301)
- 天蝎座 (294)
- 巨蟹座 (289)
- 双子座 (289)
- 处女座 (285)
- 天秤座 (276)
- 双鱼座 (268)
- 婴儿 (265)
- 水瓶座 (260)
- 射手座 (239)
- 不完美妈妈 (173)
- 跳槽那些事儿 (168)
- baby (140)
- 女婴 (132)
- 生肖 (129)
- 女儿 (129)
- 民警 (127)
- 狮子 (105)
- NBA (101)
- 家长 (97)
- 怀孕 (95)
- 儿童 (93)
- 交警 (89)
- 孕妇 (77)
- 儿子 (75)
- Angelababy (74)
- 父母 (74)
- 幼儿园 (73)
- 医院 (69)
- 童车 (66)
- 女子 (60)
- 郑州 (58)